Abstract
Two new one-dimensional chain-like compounds, K4Na4[Mn2(H2O)8Mn4(H2O)2(GeW9O34)2] · 20.5H2O (1) and K2Na4Cu2(H2O)12[Cu(H2O)2Cu4(H2O)2(SiW9O34)2] · 15H2O (2), constructed from the sandwich-type clusters, have been obtained by the routine synthetic reactions in aqueous solutions, and their structures were determined by X-ray single crystal diffraction analysis. The crystal data is following: for 1, space group, monoclinic, P 21/n, a = 16.693(3) Å, b = 14.935(3) Å, c = 20.090(4) Å, β = 92.23(3)°, V = 5004.7(17) Å3, Z = 2; For 2, space group, triclinic, P −1, a = 11.744(2) Å, b = 13.415(3) Å, c = 17.609(4) Å, α = 73.08(3)°, β = 82.68(3)°, γ = 65.18(3)°, V = 2409.1(8) Å3, Z = 1. The crystal structure of 1 shows a 1D ladder-like chain, built up of the sandwich anions [Mn4(H2O)2(GeW9O34)2]12− and the Mn2+ ions. Compound 2 is a polymeric chain, composed of the Cu-substituted sandwich-type anions [Cu4(H2O)2(SiW9O34)2]12− linked by the Cu(H2O)4 clusters. These extended materials based on the sandwich-type polyoxoanions are rarely reported in the POM chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyoxometalates (POMs), as metal–oxygen cluster ions, have been attracting extensive interests due to their enormous structural varieties and the accompanying multitude of potential applications in the field of catalysis, electrochemistry, electrochromism and magnetism [1, 2]. The evolution of POM chemistry is dependent upon the synthesis of novel polyoxoanions possessing unique structures and physicochemical versatilities. With the aim of producing novel polyoxoanions, much attention has focused on the self-assembly of preformed building units. In this subfamily, lacunary POMs represent one of the excellent inorganic precursors to construct new compounds with diverse nuclearities and structural features combined with interesting catalytic, electrochemical and magnetic properties, which have attracted considerable attention worldwidely [3]. Up to now, numerous sandwich-type polyoxoanions have been synthesized by the reactions of the lacunary polyoxoanions with the transition metal cations, and mostly belong to the well-known Weakley-, Hervé-, Krebs- and Knoth-type sandwich structures [4, 5]. They each accommodated a paramagnetic transition metal-set between two lacunary polyoxoanions, which make them exhibit interesting catalytic, magnetic, electrochemical properties. It is known to all that the sandwich-type polyoxoanions have a larger volume and a more-negative charge than that of the commonly used polyoxovanadate/-molybdate in constructing the extended structure materials, which allow the formation of higher coordination numbers with transition-metal cations. These sandwich-type clusters should be the excellent precursors, but are used scarcely for constructing the extended structure materials [6]. In this article, we reported two new one-dimensional chain-like compounds constructed from the M4-sandwiched polyoxoanions.
Experimental Section
Materials and Methods
All chemicals were commercially purchased and used without further purification. K8[β-GeW11O39] · 14H2O and K8[γ-SiW10O36] · 12H2O were synthesized according to the literatures [7] and characterized by IR spectra. Elemental analyses (H) were performed on a Perkin-Elmer 2400 CHN elemental analyzer; Si, Ge, W, Mn, Cu, Na and K were analyzed on a PLASMA-SPEC(I) ICP atomic emission spectrometer. IR spectra were recorded in the range 400–4,000 cm−1 on an Alpha Centaurt FT/IR Spectrophotometer using KBr pellets. TG analyses were performed on a Perkin-Elmer TGA7 instrument in flowing N2 with a heating rate of 10 °C min−1. Electrochemical measurements were carried out on a CHI 660A electrochemical workstation at room temperature (25–30 °C) under nitrogen atmosphere. A pHS-25B type pH meter was used for pH measurement.
Syntheses of Compounds
Synthesis of 1
K8[β-GeW11O39] · 14H2O (2.0 g, 0.61 mmol) was dissolved in 60 mL of distilled water with stirring. And then, 10 mL of 1 M MnSO4 (10 mmol) solution was added dropwise with vigorously stirring and the mixture was boiled for 4 h. After cooling to room temperature, the solution was filtrated and the filtrate was slowly evaporated at room temperature for a week, resulting in a yellow crystalline product (Yield 37% based on Ge). Anal. Calcd for 1 (%): K, 2.76; Na, 1.62; H, 1.08; Mn, 5.81; Ge, 2.56; W, 58.4; Found: K, 2.61; Na, 1.71; H, 1.19; Mn, 5.91; Ge, 2.32; W, 58.1. IR (KBr pellet): 946(m), 873(s), 765(s), 694(s), 510(w), 444(w) cm−1.
Synthesis of 2
D-Proline (0.1 g, 0.868 mmol) and CuCl2 · 2H2O (0.68 g, 3.98 mmol) were dissolved in distilled water (60 mL). The pH value of the mixture was carefully adjusted with a dilute NaOH solution (1 M) to 6.0 and then stirred for 2 h. Then 30 mL K8[γ-SiW10O36] · 12H2O (1.0 g) solution was added dropwise with vigorously stirring, and the resulting solution was heated at 90 °C for 3 h. The filtrate was kept at room temperature slow evaporation for half a month resulted in the blue crystals of 2 (yield 41% based Si). Anal. Calcd: (%): K, 1.39; Na, 1.63; H, 1.11; Cu, 7.91; Si, 1.00; W, 58.8; Found: K, 1.27; Na, 1.78; H, 1.01; Cu, 7.73; Si, 0.89; W, 58.5. IR (KBr pellet): νmax/cm−1 938(s), 888(s), 773(s), 700(s), 700(s), 489(w) and 452(w).
X-ray Crystallography
Single-crystal X-ray data for 1 and 2 was collected on a Rigaku R-AXIS RAPID IP diffractometer equipped with a normal focus 18 kW sealed tube X-ray source (Mo-Ka radiation, λ = 0.710 73 Å) operating at 50 kV and 200 mA. Data processing was accomplished with the RAXWISH processing program. A numerical absorption correction was applied. The structure was solved by direct methods and refined by full-matrix least-squares on F2 using the SHELXL 97 software [8]. All hydrogen atoms for water molecules and protonation were not located but were included in the structure factor calculations. All structures possess apparent disorders in the range of counter ions and lattice water molecules, preventing a more precise structural analysis. However, none of these deficiencies affects the structural details and reliability of the polyoxoanion structures. Their occupancies were determined by fixing the atomic displacement parameter factors (0.08) at the beginning, and then occupancy numbers were fixed to refine their atomic displacement parameter factors. Atoms with occupancies lower than 0.25 in 1 and 2 were ignored. The crystal data and structure refinements of compounds 1 and 2 are summarized in Table 1.
Results and Discussions
Structure Description
Structural Analysis of 1
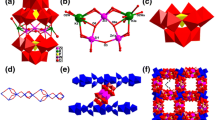
Single-crystal X-ray diffraction analyses reveal that compound 1 exhibits a 1D ladder-like chain built up of the sandwich anions [Mn4(H2O)2(GeW9O34)2]12− and the Mn2+ ions (Fig. 1). In this polymeric chain, four Mn2+ linkers coordinate to a sandwich-type anion by four μ 2-O atoms and link the two neighboring anions to constitute the 1D ladder-like chain, which was rarely reported for POM chemistry. The coordination geometry of the four linking Mn2+ ions is octahedral, and each of them is coordinated by two oxygen atoms coming from two neighboring sandwich-type [Mn4(H2O)2(GeW9O34)2]12− anions and four water molecules. The band Mn–O lengths of the four linking Mn2+ ions are in the range of 2.135(11)–2.20(2) Å. The sandwich-type polyoxoanion in this chain was composed of two [B-α-GeW9O34]10− units linked by a Mn4 cluster whose four Mn2+ ions lie in the same plane and form a centrosymmetric regular rhomblike cluster (Fig. 2), and the [B-α-GeW9O34]10− unit provides seven oxygen donor atoms (one from the central GeO4 group and one each from the six W atoms) that are capable of coordinating to the central Mn4 cluster to form the Weakley-type sandwich structure. All the W and Mn centers in the [Mn4(H2O)2(GeW9O34)2]12− cluster exhibit the octahedral coordination environments. The bond lengths of W–O are in the range of 1.717(11)–2.422(10) Å and the Mn–O bond lengths in the central cluster are in the range of 2.105(12)–2.236(12) Å.
Structural Analysis of 2
Compound 2 is composed of the sandwich-type [Cu4(H2O)2(SiW9O34)2]12− anionic moieties, Cu2+ ions, K+ cations, Na+ cations and lattice water molecules. As shown in Fig. 3, compound 2 shows a 1D chainlike structure constructed from the anions [Cu4(H2O)2(SiW9O34)2]12− and the linking {Cu(H2O)4}2+ groups. In the 1D chain, the Weakley-type polyoxoanion [Cu4(H2O)2(SiW9O34), which is isomorphic with the anion [Mn4(H2O)2(GeW9O34)2]12− observed in 1, is covalently bonded to two adjacent {Cu(H2O)4}2+ groups via two terminal-oxo atoms (Fig. 4). All W atoms in 2 exhibit the octahedral coordination environments and the W-O distances fall in the range of 1.700(13)–2.414(17) Å. All the Cu2+ ions in this 1D chain are all coordinated in a strongly distorted octahedral fashion which exhibits Jahn–Teller distortion with axial elongation. The band lengths of Cu–O of the two linking Cu2+ ions are in the range of 1.926(16)–2.429(15) Å and the bond lengths of Cu–O in the central belt of the sandwich-type anion are in the range of 1.915(16)–2.599(66) Å.
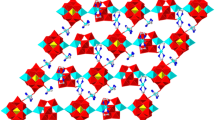
Interestingly, polymeric chains in 2 are linked by K+ into a 2D framework. Within the 2D framework, the K+ is connected with two [Cu4(H2O)2(SiW9O34)2]12− clusters, deriving from two neighboring polymeric chains to construct the 2D framework (Fig. 5). Each [Cu4(H2O)2(SiW9O34)2]12− cluster is coordinated to four K+ ions which connects with other two [Cu4(H2O)2(SiW9O34)2]12− cluster from the neighboring chains to constitute to the 2D structure. The K–O distances fall in the range of 2.77(2)–3.084(18) Å.
The oxidation states of W, Mn and Cu sites in the two compounds are determined based on the crystal color, bond lengths and angles, charge balance consideration and bond valence sum calculation [9], indicating that all W sites possess +6 oxidation states, and the manganese ions in 1 and the copper ions in 2 are all in the +2 oxidation state. Bond valence sum calculation [9] also reveals that the terminal oxygen atoms associated to the manganese ions in 1 and to the copper ions in 2 are all diprotonated.
Electrochemistry
The cyclic voltammetric behavior for 1 in a pH = 5 (0.4 M CH3COONa + CH3COOH) buffer solution exhibits four reduction peaks in the potential range of +1.3 to −1.2 V and the mean peak potentials are 0.616, −0.225, −0.911 and −1.025 V (vs. Ag/AgCl), respectively (see Fig. 6). The first two reduction waves located at 0.616 and −0.225 V and one single oxidation process (0.972 V) are attributed to the redox processes of the Mnα centers [10]. The peaks at −0.911 and −1.025 V are ascribed to the reduction process of Wε centers [11]. Figure 7 shows the typical cyclic voltammetric behavior of compound 2 in the pH = 5 (0.4 M CH3COONa + CH3COOH) buffer solution at the scan rate of 10 mV s−1. It can be seen that in the potential range +0.4 to −0.9 V, four reduction peaks appear and the mean peak potentials are −0.041, −0.103, −0.409 and −0.691 V (vs. Ag/AgCl), respectively. The last two redox process corresponds to the redox of the Wε atoms in the polyoxoanion framework and the domain where the wave located at was also observed in the other W-containing POMs [11]. The first two reduction waves and their oxidation counterpart, a single oxidation process located at +0.068 V, are attributed to the redox processes of the Cu2+ centers. The two reduction waves located at −0.041 and −0.103 V features the two-step reduction of Cu2+ to Cu0 through Cu1+ [12].
TG Analyses
In order to examine the thermal stability of compounds 1 and 2, thermal gravimetric (TG) analyses were carried out for 1 and 2. Thermogravimetric (TG) curve of 1 (Fig. S1) shows a total weight loss of 10.4% from 33 to 465 °C, attributed to the loss of all lattice and coordinated water molecules in 1 (calcd 9.68%). The thermal gravimetric (TG) curve of 2 shows a weight loss of 11.8% in the range of 28–460 °C, which corresponds to the loss of all noncoordinated and coordinated water molecules (Fig. S2). The weight loss of 11.8% is a little higher than the calculated value of 9.92%. The reason could be that the solvent accessible voids of about 199.1 Å3 (as determined by PLATON program) exist in the unit cell of compound 1, which could accommodate several disordered water molecules in the formula unit.
Conclusions
In conclusion, two new 1D heteropolyoxotungstates built up of the sandwich-type clusters and transition metal linkers, K4Na4[Mn2(H2O)8Mn4(H2O)2(GeW9O34)2] · 20.5H2O (1) and K2Na4Cu2(H2O)12[Cu(H2O)2Cu4(H2O)2(SiW9O34)2] · 15H2O (2), have been obtained in the aqueous solutions. The successful synthesis of the two compounds suggests that the sandwich-type polyoxoanions might be the useful precursors for the construction of POM-based extended framework. The properties and functionalities of such compounds might be adjusted by the choice of different transition metal ions in the sandwich sites. More work in this field is underway.
Supplementary Materials
X-ray crystallographic files in CIF format, TG curves and the IR spectra of compounds 1 and 2. Further details on the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: +49-7247-808-666; e-mail: crysdata@fiz-karlsruhe.de), on quoting the depository numbers CSD-418859 for 1 and CSD-418860 for 2.
References
(a) M. T. Pope, Heteropoly and Isopoly Oxometalates (Springer, Berlin, 1983); (b) J. S. Anderson (1937). Nature 140, 850; (c) C. L. Hill (1998). Chem. Rev. 98, 1; (d) L. C. Baker and D. C. Glick (1998). Chem. Rev. 98, 3; (e) A. Müller, S. Q. N. Shah, H. Bögge, and M. Schmidtmann (1999). Nature 397, 48; (f) E. Coronado and G. García (1998). Chem. Rev. 98, 273; (g) D. L. Long, E. Burkholder, and L. Cronin (2007). Chem. Soc. Rev. 36, 105.
(a) H. Y. An, E. B. Wang, D. R. Xiao, Y. G. Li, Z. M. Su, and L. Xu (2006). Angew. Chem. Int. Ed. 45, 904; (b) Q. Li, Y. G. Wei, J. Hao, Y. L. Zhu, and L. S. Wang (2007). J. Am. Chem. Soc. 129, 5810; (c) C. Streb, C. Ritchie, D. L. Long, P. Kögerler, and L. Cronin (2007). Angew. Chem. Int. Ed. 46, 7579.
(a) S. T. Zheng, D. Q. Yuan, H. P. Jia, J. Zhang, and G. Y. Yang (2007). Chem. Commun. 1858; (b) Z. M. Zhang, Y. G. Li, E. B. Wang, X. L. Wang, C. Qin, and H. Y. An (2006). Inorg. Chem. 45, 4313; (c) K. Fukaya and T. Yamase (2003). Angew. Chem. Int. Ed. 42, 654; (d) A. Müller, M. T. Pope, A. M. Todea, H. Bögge, J. van Slageren, M. Dressel, P. Gouzerh, R. Thouvenot, B. Tsukerblat, and A. Bell (2007). Angew. Chem. Int. Ed. 46, 4477; (e) S. S. Mal and U. Kortz (2005). Angew. Chem. Int. Ed. 44, 3777; (f) C. Pichon, P. Mialane, A. Dolbecq, J. Marrot, E. Rivière, B. Keita, L. Nadjo, and F. Sécheresse (2007). Inorg. Chem. 46, 5292; (g) Z. M. Zhang, Y. F. Qi, Qin, Y. G. Li, E. B. Wang, X. L. Wang, Z. M. Su, and L. Xu (2007). Inorg. Chem. 46, 8162; (h) Z. M. Zhang, E. B. Wang, Y. F. Qi, Y. G. Li, B. D. Mao, and Z. M. Su (2007). Cryst. Growth. Des. 7, 1305; (i) Z. M. Zhang, S. Yao, Y. G. Li, Y. H. Wang, Y. F. Qi, and E. B. Wang (2007). Chem. Commun. (2008) in press (DOI: 10.1039/b718374g).
(a) T. J. R. Weakley, H. T. Evans Jr., J. S. Showell, T G. Fourné, and C. M. Tourné (1973). J. Chem. Soc. Chem. Commun. 139; (b) R. G. Finke, M. Droege, J. R. Hutchinson, and O. Gansow (1981). J. Am. Chem. Soc. 103, 1587; (c) S. H. Wasfi, A. L. Rheingold, G. F. Kokoszka, and A. S. Goldstein (1987). Inorg. Chem. 26, 2934; (d) Z. M. Zhang, E. B. Wang, Y. G. Li, Y. F. Qi, and H. Q. Tan (2007). J. Mol. Struct. 843, 128; (e) X. Zhang, Q. Chen, D. C. Duncan, R. J. Lachicotte, and C. L. Hill (1997). Inorg. Chem. 36, 4381; (f) J. P. Wang, X. Y. Duan, X. D. Du, and J. Y. Niu (2006). Cryst. Growth. Des. 6, 2266.
(a) M. Bösing, A. Nöh, I. Loose, and B. Krebs (1998). J. Am. Chem. Soc. 120, 7252; (b) P. Mialane, J. Marrot, E. Rivière, J. Nebout, and G. Hervé (2001). Inorg. Chem. 40, 44; (c) F. Xin and M. T. Pope (1996). J. Am. Chem. Soc. 118, 7731; (d) N. Laronze, J. Marrot, and G. Hervé (2003). Inorg. Chem. 42, 5857; (e) P. T. Witte, S. R. Chowdhury, J. E. Elshof, D. Sloboda-Rozner, and R. N. P. L. Alsters (2005). Chem. Commun. 1206; (f) L. H. Bi, U. Kortz, B. Keita, L. Nadjo, and H. Borrmann (2004). Inorg. Chem. 43, 8367; (g) L. Ruhlmann, J. Canny, R. Contant, and R. Thouvenot (2002). Inorg. Chem. 41, 3811.
(a) S. T. Zheng, M. H. Wang, and G. Y. Yang (2007). Chem. Asian J. 2, 1380; (b) Z. M. Zhang, J. Liu, E. B. Wang, C. Qin, Y. G. Li, Y. F. Qi, and X. L. Wang (2008). Dalton Trans. 463; (c) J. P. Wang, X. D. Du, and J. Y. Niu (2006). Chem. Lett. 35, 1408; (d) U. Kortz, S. Nellutla, A. C. Stowe, N. S. Dalal, J. V. Tol, and B. S. Bassil (2004). Inorg. Chem. 43, 144.
(a) A. Tézé and G. Hervé (1990). Inorg. Synth. 27, 85; (b) J. Canny, A. Tézé, R. Thouvenot, and G. Hervé (1986). Inorg. Chem. 25, 2114; (c) N. H. Nsouli, B. S. Bassil, M. H. Dickman, U. Kortz, B. Keita, and L. Nadjo (2006). Inorg. Chem. 45, 3858.
(a) G. M. Sheldrick, SHELXS97, Program for Crystal Structure Solution (University of Gcttingen, Germany, 1997); (b) G. M. Sheldrick, SHELXL97, Program for Crystal Structure Refinement (University of Gcttingen, Germany, 1997).
The valence sum calculations are performed on a program of bond valence calculator, version 2.00 February (1993), written by C. Hormillosa with assistance from S. Healy, distributed by I. D. Brown.
B. Keita, P. Mialane, F. Sécheresse, P. Oliveira, and L. Nadjo (2007). Electrochem. Commun. 9, 164.
(a) B. S. Bassil, U. Kortz, A. S. Tigan, J. M. Clemente-Juan, B. Keita, P. Oliveira, and L. Nadjo (2005). Inorg. Chem. 44, 9360; (b) I. M. Mbomekalle, B. Keita, M. Nierlich, U. Kortz, P. Berthet, and L. Nadjo (2003). Inorg. Chem. 42, 5143.
(a) S. Nellutla, J. van Tol, N. S. Dalal, L. H. Bi, U. Kortz, B. Keita, L. Nadjo, G. A. Khitrov, and A. G. Marshall (2005). Inorg. Chem. 44, 9795; (b) B. Keita, I. M. Mbomekalle, and L. Nadjo (2003). Electrochem. Commun. 5, 830.
Acknowledgements
This work was supported by National Science Foundation of China (No. 20371011), Analysis and Testing Foundation of Northeast Normal University, Ph.D station Foundation of Ministry of Education (No. 20060200002).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gan, X., Zhang, Z., Yao, S. et al. Two New One-Dimensional Chain-Like Compounds Constructed from the Sandwich-Type Polyoxotungstate Clusters. J Clust Sci 19, 401–410 (2008). https://doi.org/10.1007/s10876-008-0183-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-008-0183-8