Abstract
Purpose
Patients with auto-antibodies neutralizing type I interferons (anti-IFN auto-Abs) are at risk of severe forms of coronavirus disease 19 (COVID-19). The chest computed tomography (CT) scan characteristics of critically ill COVID-19 patients harboring these auto-Abs have never been reported.
Methods
Bicentric ancillary study of the ANTICOV study (observational prospective cohort of severe COVID-19 patients admitted to the intensive care unit (ICU) for hypoxemic acute respiratory failure between March 2020 and May 2021) on chest CT scan characteristics (severity score, parenchymal, pleural, vascular patterns). Anti-IFN auto-Abs were detected using a luciferase neutralization reporting assay. Imaging data were collected through independent blinded reading of two thoracic radiologists of chest CT studies performed at ICU admission (± 72 h). The primary outcome measure was the evaluation of severity by the total severity score (TSS) and the CT severity score (CTSS) according to the presence or absence of anti-IFN auto-Abs.
Results
Two hundred thirty-one critically ill COVID-19 patients were included in the study (mean age 59.5 ± 12.7 years; males 74.6%). Day 90 mortality was 29.5% (n = 72/244). There was a trend towards more severe radiological lesions in patients with anti-IFN auto-Abs than in others, not reaching statistical significance (median CTSS 27.5 (21.0–34.8) versus 24.0 (19.0–30.0), p = 0.052; median TSS 14.5 (10.2–17.0) versus 12.0 (9.0–15.0), p = 0.070). The extra-parenchymal evaluation found no difference in the proportion of patients with pleural effusion, mediastinal lymphadenopathy, or thymal abnormalities in the two populations. The prevalence of pulmonary embolism was not significantly different between groups (8.7% versus 5.3%, p = 0.623, n = 175).
Conclusion
There was no significant difference in disease severity as evaluated by chest CT in severe COVID-19 patients admitted to the ICU for hypoxemic acute respiratory failure with or without anti-IFN auto-Abs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory distress syndrome virus 2 (SARS-CoV-2) infection leads to a broad spectrum of manifestations with vast inter-individual variability, ranging from asymptomatic presentations to severe coronavirus disease 2019 (COVID-19)-associated acute respiratory distress syndrome (ARDS) requiring intensive care unit (ICU) admission in 5–10% of cases [1]. The protective role of type I interferons (IFNs) immunity during SARS-CoV-2 infection was documented by the observation of life-threatening COVID-19 pneumonia in patients with inborn errors of immunity affecting Toll-like receptor 3 (TLR3) or TLR7-dependent type I IFNs induction and amplification, in 1–5% of cases of critical COVID-19 pneumonia [2, 3]. Auto-immune phenocopy of inborn errors of type I IFN-dependent immunity were also shown to underlie life-threatening COVID-19 pneumonia. Circulating IgG auto-antibodies (Abs) neutralizing IFN-α2 and/or IFN-ω (10 ng/mL) were found in 10% of critical COVID-19 cases in an international cohort, as compared with 0% of mildly/asymptomatic cases and 0.3% of uninfected individuals [4]. Auto-Abs neutralizing type I IFNs [4,5,6] are now established as risk factors of developing severe COVID-19 in the general population and have been repeatedly found to have a prevalence of around 10% among critically ill COVID-19 patients [7]. Beyond these epidemiological data, which have been replicated in more than 30 studies, there are several lines of evidence supporting the fact that auto-Abs against type I IFNs are strong biological determinants associated with a risk of severe COVID-19 [6]: (1) the auto-Abs neutralize the antiviral activity of type I IFNs against SARS-CoV-2 in vitro [4]; (2) they were found in the blood and respiratory tract of patients [8]; (3) a key virulence factor of SARS-CoV-2 is its capacity to impair type I IFN response [9]; and (4) animals with type I deficiency develop critical diseases [10]. Surprisingly, in a recent multicenter study of critically ill COVID-19 patients, the presence of auto-Abs neutralizing type I IFN was not associated with outcome [11]. Such clinical observation was consistent with the previous finding that critically ill patients exhibit a deficient type I IFN-stimulated gene (ISG) response in myeloid cells, whether they harbor auto-Abs or not [12, 13].
The chest computed tomography (CT) characteristics of critically ill COVID-19 patients with auto-Abs neutralizing type I IFN have, to the best of our knowledge, not been studied. Chest CT has been widely used as a diagnostic, prognostic, and phenotyping tool, as well as a detection method for pulmonary thromboses associated with COVID-19 [14, 15]. In this ancillary analysis of an observational prospective cohort study, we aimed at studying the chest CT scans of patients with or without auto-Abs neutralizing type I IFN.
Methods
Study Design and Participants
This is an ancillary study of the ANTICOV study [11], an observational prospective French multicenter study (NCT04733105), which included patients between March 31, 2020, and May 1, 2021. This ancillary study included patients from the medical and surgical ICUs of Henri Mondor Hospital (Créteil, France). Inclusion criteria were as follows: age ≥ 18 years, SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR), patient admitted in the ICU for acute respiratory failure (SpO2 ≤ 90% and need for supplemental oxygen or any kind of ventilator support), chest CT performed at ICU admission ±72 h. The study was approved by the Comité de Protection des Personnes Nord-Ouest IV (N° EudraCT/ID-RCB: 2020-A03009-30). Informed consent was obtained from all patients or their relatives.
Demographics, clinical, and laboratory variables were recorded upon ICU admission and during ICU stay in the original cohort. Additional data pertaining to thoracic medical history and exposure to pneumotoxic drugs relevant to guide CT interpretation were retrospectively gathered from the patients’ medical files. The severity of the disease upon ICU admission was assessed using the World Health Organization (WHO) 10-point progression scale [16] and the sequential organ failure assessment (SOFA score) [17].
Evaluation of Anti-interferon Auto-antibodies by Luciferase Reporter Assays
Auto-Abs positivity was assessed on serum samples collected during the first week of ICU admission. The blocking activity of anti-IFN-α2 and anti-IFN-ω auto-Abs was determined with a reporter luciferase activity, as previously described [5]. Briefly, HEK293T cells were transfected with a plasmid containing the Firefly luciferase gene under the control of the human interferon-stimulating response element (ISRE) promoter in the pGL4.45 backbone, and a plasmid constitutively expressing Renilla luciferase for normalization (pRL-SV40). Cells were transfected in the presence of the X-tremeGene9 transfection reagent (Sigma-Aldrich, ref. number 6365779001) for 24 hours. Cells in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific) supplemented with 2% fetal calf serum (FCS) and 10% healthy control or patient serum (after inactivation at 56 °C, for 20 min) were either left unstimulated or were stimulated with IFN-α2 (Milteny Biotec, ref. number 130-108-984), IFN-ω (Merck, ref. number SRP3061), at 10 ng/mL or 100 pg/mL, or IFN-β (Milteny Biotech, ref. number: 130-107-888) at 10 ng/mL, for 16 h at 37 °C. Each sample was tested once for each cytokine and dose. Finally, cells were lysed for 20 min at room temperature and luciferase levels were measured with the Dual-Luciferase® Reporter 1000 assay system (Promega, ref. number E1980), according to the manufacturer’s protocol. Luminescence intensity was measured with a VICTOR-X Multilabel Plate Reader (PerkinElmer Life Sciences, USA). Firefly luciferase activity values were normalized against Renilla luciferase activity values. These values were then normalized against the median induction level for non-neutralizing samples and expressed as a percentage. Samples were considered neutralizing if luciferase induction, normalized against Renilla luciferase activity, was below 15% of the median values for controls tested the same day. In this study, having any neutralizing auto-Abs regardless of its specificity was considered as positive for anti-IFN auto-Abs.
Computed Tomography Assessment
Protocol for chest CT acquisition of COVID-19 patients was as follows: helicoidal volumic acquisition from pulmonary apex to iliac crest, on a Revolution (General Electric, USA) CT machine, after automatic adaptation of mAs and kV. CT pulmonary angiogram was performed if there was clinical suspicion of pulmonary embolism or for patients requiring > 3L/min of oxygen supplementation, using a dual-energy CT (80 kV and 140 kV) acquisition protocol after injection of iodine contrast media (IOMERON 350, Bracco, Fr). Images with pulmonary filter were systematically reconstructed.
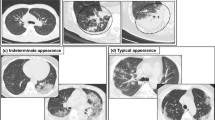
Interpretation was performed independently by two radiologists with 5 and 8 years of experience in thoracic imaging (Y.Z. and S.M.), blinded for anti-IFN Abs results. A systematic analysis was conducted based on a predefined reading grid. Representative examples of CT patterns are shown in Fig. 1.
Representative panels of studied lung patterns. Axial views are shown except for the H panel. A Mixed distribution (central and peripheral) hazy ground glass opacities; B pseudo-nodular ground glass opacities; C peripheral linear consolidations; D bilateral posterior condensed consolidations; E bilateral peripheral crazy paving; F bronchiectasis; G posterobasal honeycombing; H coronal view of both ground glass opacities and consolidations with a mixed distribution
Primary outcome measures: for severity assessment, we used two scores developed for COVID-19 patients and based on semi-quantitative topographic evaluation of affected lung. The total severity score (TSS [18]) relies on the visual scoring from 0 (no involvement) to 4 (severe, > 75% involvement) of lesions in the five lung lobes, adding to a total value of 0 to 20. The CT severity score (CTSS [19]) follows the same principle, dividing the lungs in 20 regions, each score from 0 to 2, with a result ranging from 0 to 40. Cutoff value for severity is 19. Discrepancies were resolved by a second reading by both radiologists.
Statistics
Statistical analysis was performed using the R software (R Foundation for Statistical Computing, Vienna, Austria). A Mann-Whitney test was used to compare continuous variables and a chi2 test was used for categorical variables (or a Fischer exact test when chi2 requirements were not met). Two-tailed p-values < 0.05 were considered statistically significant.
Results
Clinical Characteristics and Outcomes of the Patients
During the study period, there were 410 patients admitted in the ICUs at Henri Mondor Hospital who met inclusion criteria for the ANTICOV study. Clinical characteristics of patients included in the cohort (n = 390) according to their anti-IFN auto-Abs status are available in Table S1. There were no statistically significant differences in demographic, comorbidities, admission data, or day 90 mortality between patients who had circulating anti-IFN auto-Abs (12.3%; n = 48/390) or not (87.7%; n = 342/390).
Among them, 244 (59%) patients had a chest CT scan (n = 175 (76%) with pulmonary angiogram) obtained in the 72-h time frame around ICU admission, including 231 patients (95%) who had anti-IFN auto-Abs results available, and who constituted the final study population (Fig. 2).
Population characteristics are shown in Table 1. Most common comorbidities were hypertension and diabetes in 109 (44.7%) and 69 (28.3%) patients, respectively. Immunosuppression and history of relevant intrathoracic disease were found in less than 5% of patients. Median time (interquartile range, IQR) between first symptoms and ICU admission was 9 (6–12) days, with no significant difference between patients having auto-Abs or not (Table S1), and time between first symptoms and chest CT scan acquisition was 8 (6–11) days. WHO severity scale was 6 (6–8). Thirty patients (13%) had a positive result for anti-IFN auto-Abs, consistent with previous findings [4]. Day 90 mortality was 29.5% (n = 72/244).
Comparison between patients with or without a chest CT study available yielded no significant difference for most of demographic and admission characteristics (Table S2, online supplement). However, there were statistically significant differences regarding the frequency of several comorbidities (i.e., body mass index, asthma, hypertension, history of intrathoracic neoplasia) possibly related to COVID-19 severity at admission, with also higher WHO severity scale, and SOFA scores in patients who had no chest CT scan performed than in others. There was also a higher proportion of patients receiving ECMO in patients without a chest CT, as expected given the risks of performing CT scan in unstable patients (Table S1).
Chest CT Scan Patterns of Critically Ill COVID-19 Patients
CT characteristics of severe COVID-19 patients displayed bilateral opacities in 99.1% of cases (n = 229/244), with ground glass opacities in 97.4% (n = 225/244) of them (Table 2). Severity scores were high with a median (IQR) CTSS of 24.0 (19.0–30.0) and TSS of 12.0 (9.0–15.0). Consolidations and interstitial changes were found in 80.5% (n = 186/244) and 22.5% (n = 52/244) of patients, respectively. The most frequent interstitial change was bronchiectasis in 20.3% (n = 47/244) of patients. Finally, the prevalence of pulmonary embolism in the subset of patients who had a CT pulmonary angiogram study performed was 5.7% (n = 10/175).
Chest CT Scan Patterns of Critically Ill COVID-19 Patients with Positive Anti-IFN Auto-Abs
Regarding severity scores, there was a trend towards a more severe disease in patients with auto-IFN anti-Abs than in others, though not reaching statistical significance (median CTSS 27.5 (21.0–34.8) versus 24.0 (19.0–30.0), p = 0.052; median TSS 14.5 (10.2–17.0) versus 12.0 (9.0–15.0), p = 0.070). Comparison of the two groups (Table 2) showed no statistically significant difference for ground glass opacities (96.7% versus 97.5%, p0.570), its distribution, or its aspect. Similarly, there was no difference in terms of prevalence or aspect of parenchymal interstitial changes. Alveolar infiltrates were observed in similar proportions in patients with or without anti-IFN auto-Abs (73.3% vs 81.6%, p = 0.323), but with a pattern distribution being statistically different between the two populations (6.7% versus 27.9% of linear and 66.7 versus 54.2% of condensed consolidation, p = 0.025 for pattern distribution comparison). The extra-parenchymal evaluation found no difference in the proportion of patients with or without pleural effusion (23.3% versus 17.4%, p = 0.449), lymphadenopathy (50% versus 42.3%, p = 0.437), or pulmonary embolism prevalence between the two populations (8.7% versus 5.3%, p = 0.623, n = 175). The prevalence of thymic abnormalities was not significantly different between groups (26.7% versus 19.9%, p = 0.468). Because thymus might be involved in auto-immunity processes, we further explored the clinical characteristics and outcomes of the 8 patients with thymic abnormalities and auto-IFN anti-Abs (Table 3) and found no patient who developed a thymoma or a myasthenia during follow-up. Yet, one additional patient, who could not be included in the cohort because he had a chest CT scan performed outside the predefined time window (i.e., 6 days before ICU admission), presented a histologically confirmed thymoma (Table 3).
Discussion
During the pandemic, the search for risks factors of severe forms of COVID-19 beyond the rapidly described demographic and clinical factors such as age, sex, hypertension, and overweight has led to the identification of innate or acquired genetic or immunological predispositions [2, 4]. Among those, the presence of circulating anti-IFN auto-Abs has been demonstrated to be associated with a higher risk of severe COVID-19 and mortality in the general population and in patients with mild disease [6, 20]. This was consistent with the identification of inborn errors of TLR3- or TLR7-dependent type I IFN immunity [2, 3, 21,22,23]. However, their impact on mortality is less clear in COVID-19 patients already admitted in the ICU [11]. To our knowledge, this is the first study of thoracic imaging of patients with anti-IFN auto-Abs admitted in the ICU for severe COVID-19.
Patients with anti-IFN auto-Abs showed a non-significant trend towards more severe and extensive lesions during the early phase of COVID-19-associated acute respiratory failure. Alveolar infiltrates were more frequently condensed than linear, possibly reflecting a more severe disease. These findings are consistent with the literature given the association, on the first hand, between these antibodies, the severity of COVID-19 [6] and oxygen supplementation requirement at ICU admission [11], and, on the other hand, between the extent of pulmonary infiltrate on chest CT and disease severity [15]. Indeed, we could expect pulmonary inflammation to be exacerbated in anti-IFN auto-Abs patients who are lacking competent innate immunity to control viral replication [24]. However, ground glass opacities, which have been shown to be a surrogate of inflammatory lung injury [25], were not different between groups. This finding is in line with a recent study of severe COVID-19 patients showing no significant different broncho-alveolar fluid concentration of inflammatory markers in patients harboring anti-IFN auto-Abs than in those who did not [8]. These results are consistent with the hypothesis of a common pathogenesis for critical COVID-19 involving impaired type I IFN responses in all patients, as suggested by the similarities between patients with and without anti-IFN auto-Abs [13], and are in line with the previously suggested hypothesis of a global emerging framework of an IFN I deficiency causal for critical COVID-19, where anti-IFN auto-Abs are only one of the known and unknown mechanisms for this deficiency [22].
There was also no significant difference in the prevalence of pulmonary embolism (PE) in patients with versus without anti-IFN auto-Abs. The following factors should however be taken into account to interpret these findings: (1) CT pulmonary angiograms were not routinely performed, especially in the more severe patients potentially leading to a selection bias; (2) The overall 5.7% PE rate in our cohort was lower than that reported in historical series (23.3 to 29.6% in a meta-analysis [14]) reducing our statistical power and our ability to detect between-group differences. We did not find specific data on anti-IFN auto-Abs and thrombosis during severe COVID-19 but the relationship between innate immunity, inflammation, and coagulation has been well described [26], and might be specifically relevant given the endothelial tropism of SARS-CoV-2 [27].
The origin of anti-IFN auto-Abs remains unknown but there is a hypothesis about the role of the thymus in their genesis [20, 28]. This hypothesis is backed up by observations in other human diseases, such as the link between auto-antibodies-mediated myasthenia gravis and thymoma [29]. Of note, most of the patients with auto-immune polyendocrine syndrome type 1 (APS-1), a disease characterized by the loss of thymic central immune-tolerance, carry anti-IFN auto-Abs [30]; these patients are at risk for severe COVID-19 [4]. Moreover, the loss of immune-tolerance accompanying thymic aging [31] might account for the increasing prevalence of anti-IFN auto-Abs with age [5]. In our study, however, there was no significant difference regarding the presence of thymic abnormalities between patients having anti-IFN auto-Abs or not, a finding that might argue against thymic involvement in the genesis of these auto-Abs. While none of 8 reported patients included in the cohort developed thymoma at latest known follow-up, we report one additional patient who developed a histologically confirmed thymoma, suggesting that in rare instances there might be an association between thymoma and anti-IFN auto-Abs.
Our study has several limitations. First, it included only two centers, making it susceptible to selection biases (tertiary care centers) and different care practices limiting its external validity. However, chest CT scans analyzed in the current study were obtained early in the hospital stay so that the impact of management strategies on CT scan patterns was probably low. Including the patients from the whole cohort might have helped generalizing the results but would have implied transferring CT scan images from other centers. Second, the ancillary part of this study was retrospective and CT studies were not performed routinely in all patients. Populations of patients with and without CT data available were largely comparable, suggesting the risk of selection bias was low. Yet, we acknowledge that patients who underwent a chest CT scan were less severe than others (i.e., they had lower severity of illness scores and required less frequent extra-corporeal membrane oxygenation support), reflecting the fact that the most unstable patients could not be transported. Third, the 72-h time frame around ICU admission for CT acquisition is debatable, as COVID-19 is well known for its dynamic evolution in two phases (replicative and inflammatory) [24]. Because a longitudinal follow-up was impossible, this time window was arbitrarily chosen to capture the early phase of severe SARS-CoV-2 infection and avoid the risks of chest CT pattern changes attributable to the ICU stay. In contrast, including only earlier chest CT scans obtained at hospital admission would have significantly lowered the number of patients included and thus our capacity to show between-group differences. On the other hand, we can speculate this might have increased the contrast between patients having auto-Abs or not as the former might have shown more chest CT images than the latter at an earlier stage of the disease, in the context of a more impaired type I IFN response associated with higher viral load and lung inflammation.
Our study also has strengths, including the relatively large number of critically ill patients included, having been screened for anti-IFN auto-Abs and chest CT scan, and the independent blinded interpretation protocol of chest CT scan by chest expert radiologists.
In conclusion, the presence of auto-Abs against type I IFNs in critically ill patients with severe COVID-19 was not associated with significant differences in chest CT severity scores.
Data Availability
Original data presented in the manuscript are available on reasonable request to the corresponding author.
Abbreviations
- Ab/Abs:
-
Antibody/antibodies
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- ICU:
-
Intensive care unit
- IFN:
-
Interferon
- IQR:
-
Interquartile range
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SD:
-
Standard deviation
References
COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73.
Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570.
Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6:eabl4348.
Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585.
Bastard P, Gervais A, Voyer TL, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~ 4% of uninfected individuals over 70 years and account for ~ 20% of COVID-19 deaths. Sci Immunol. 2021;19:eabl4340.
Manry J, Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, et al. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc Natl Acad Sci USA. 2022;119:e2200413119.
Wang X, Tang Q, Li H, Jiang H, Xu J, Bergquist R, et al. Autoantibodies against type I interferons in COVID-19 infection: a systematic review and meta-analysis. Int J Infect Dis. 2023;130:147–52.
Philippot Q, Fekkar A, Gervais A, Le Voyer T, Boers LS, Conil C, et al. Autoantibodies neutralizing type I IFNs in the bronchoalveolar lavage of at least 10% of patients during life-threatening COVID-19 pneumonia. J Clin Immunol. 2023;43:1093–103.
Leslie M. A viral arsenal. Science. 2022;378:128–31.
Israelow B, Song E, Mao T, Lu P, Meir A, Liu F, et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med. 2020;217:e20201241.
Arrestier R, Bastard P, Belmondo T, Voiriot G, Urbina T, Luyt C-E, et al. Auto-antibodies against type I IFNs in > 10% of critically ill COVID-19 patients: a prospective multicentre study. Ann Intensive Care. 2022;12:121.
Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.
Van Der Wijst MGP, Vazquez SE, Hartoularos GC, Bastard P, Grant T, Bueno R, et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med. 2021;13:eabh2624.
Tan BK, Mainbourg S, Friggeri A, Bertoletti L, Douplat M, Dargaud Y, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021;76:970–9.
Kanne JP, Bai H, Bernheim A, Chung M, Haramati LB, Kallmes DF, et al. COVID-19 Imaging: what we know now and what remains unknown. Radiology. 2021;299:E262–79.
Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–7.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol. 2020;30:4407–16.
Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q, et al. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol Cardiothorac Imaging. 2020;2:e200047.
Puel A, Bastard P, Bustamante J, Casanova J-L. Human autoantibodies underlying infectious diseases. J Exp Med. 2022;219:e20211387.
Zhang Q, Bastard P, Human Genetic Effort COVID, Karbuz A, Gervais A, Tayoun AA, et al. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature. 2022;603:587–98.
Su HC, Jing H, Zhang Y, Casanova J-L. Interfering with interferons: a critical mechanism for critical COVID-19 pneumonia. Annu Rev Immunol. 2023;41:561–85.
Matuozzo D, Talouarn E, Marchal A, Zhang P, Manry J, Seeleuthner Y, et al. Rare predicted loss-of-function variants of type I IFN immunity genes are associated with life-threatening COVID-19. Genome Med. 2023;15:22.
Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–42.
Remy-Jardin M, Giraud F, Remy J, Copin MC, Gosselin B, Duhamel A. Importance of ground-glass attenuation in chronic diffuse infiltrative lung disease: pathologic-CT correlation. Radiology. 1993;189:693–8.
Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–30.
Higashikuni Y, Liu W, Obana T, Sata M. Pathogenic basis of thromboinflammation and endothelial injury in COVID-19: current findings and therapeutic implications. IJMS. 2021;22:12081.
Bastard P, Zhang Q, Zhang S-Y, Jouanguy E, Casanova J-L. Type I interferons and SARS-CoV-2: from cells to organisms. Curr Opin Immunol. 2022;74:172–82.
Marx A, Yamada Y, Simon-Keller K, Schalke B, Willcox N, Ströbel P, et al. Thymus and autoimmunity. Semin Immunopathol. 2021;43:45–64.
Meager A, Visvalingam K, Peterson P, Möll K, Murumägi A, Krohn K, et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289.
Srinivasan J, Lancaster JN, Singarapu N, Hale LP, Ehrlich LIR, Richie ER. Age-related changes in thymic central tolerance. Front Immunol. 2021;12:676236.
Acknowledgements
We thank the patients and their families for placing their trust in us. We warmly thank the members of both branches of the Laboratory of Human Genetics of Infectious Diseases. We warmly thank Y. Nemirovskaya, M. Woollett, D. Liu, S. Boucherit, C. Rivalain, M. Chrabieh, and L. Lorenzo for administrative assistance.
Funding
Nicolas de Prost received a grant from the Agence Nationale de la Recherche (Résilience COVID-19: ANR-21-COVR-0022). The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364 and R01AI163029), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, Mercatus Center at George Mason University, the Fisher Center for Alzheimer’s Research Foundation, the Meyer Foundation, the JPB Foundation, the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the ANRS-COV05, ANR GENVIR (ANR-20-CE93-003),ANR AABIFNCOV (ANR-20-CO11-0001) and ANR GenMISC (ANR-21-COVR-0039) projects, the European Union’s Horizon 2020 research and innovation programme under grant agreement No 824110 (EASI-genomics), the Square Foundation, Grandir - Fonds de solidarité pour l’enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, The French Ministry of Higher Education, Research, and Innovation (MESRI-COVID-19), Institut National de la Santé et de la Recherche Médicale (INSERM), REACTing-INSERM, and the University of Paris. The study was supported by the ORCHESTRA project which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 101016167. P.B. was supported by the French Foundation for Medical Research (FRM, EA20170638020) and by the MD-PhD program of the Imagine Institute (with the support of the Fondation Bettencourt-Schueller). P.B. was supported by a “Poste CCA-INSERM-Bettencourt” (with support from the Bettencourt-Schueller Foundation) and the FRM (EA20170638020).
Author information
Authors and Affiliations
Contributions
B.L.R., Y.Z., S.M., and NdP. designed the study, collected and analyzed the data, and wrote the manuscript. B.L.R., Y.Z., S.M., and NdP. analyzed the data and reviewed the manuscript. P.B. and J.L.C. performed laboratory measurements of auto-antibodies against type I IFN and antinuclear auto-antibodies and reviewed the manuscript. B.P. performed the statistical analyses and reviewed the manuscript. R.A., R.B., and A.M.D. collected clinical data and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Comité de Protection des Personnes Nord-Ouest IV (N° EudraCT/ID-RCB: 2020-A03009-30).
Consent to Participate
Informed consent was obtained from all patients or their relatives.
Consent to Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lafont Rapnouil, B., Zaarour, Y., Arrestier, R. et al. Chest Computed Tomography Characteristics of Critically Ill COVID-19 Patients with Auto-antibodies Against Type I Interferons. J Clin Immunol 44, 15 (2024). https://doi.org/10.1007/s10875-023-01606-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10875-023-01606-4