Abstract
Purpose
About 20–30% of patients with common variable immunodeficiency (CVID) develop granulomatous-lymphocytic interstitial lung disease (GLILD) as one of several non-infectious complications to their immunodeficiency. The purpose of this study was to identify biomarkers that could distinguish GLILD from other non-infectious complications in CVID.
Methods
We analyzed serum biomarkers related to inflammation, pulmonary epithelium injury, fibrogenesis, and extracellular matrix (ECM) remodeling, and compared three subgroups of CVID: GLILD patients (n = 16), patients with other non-infectious complications (n = 37), and patients with infections only (n = 20).
Results
We found that GLILD patients had higher levels of sCD25, sTIM-3, IFN-γ, and TNF, reflecting T cell activation and exhaustion, compared to both CVID patients with other inflammatory complications and CVID with infections only. GLILD patients also had higher levels of SP-D and CC16, proteins related to pulmonary epithelium injury, as well as the ECM remodeling marker MMP-7, than patients with other non-infectious complications.
Conclusion
GLILD patients have elevated serum markers of T cell activation and exhaustion, pulmonary epithelium injury, and ECM remodeling, pointing to potentially important pathways in GLILD pathogenesis, novel targets for therapy, and promising biomarkers for clinical evaluation of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common variable immunodeficiency (CVID) is the most common symptomatic primary immunodeficiency in adults with a prevalence of 1:25,000–1:50,000 [1]. The patients are characterized by decreased levels of IgG and IgA with or without low IgM levels, with poor antibody response to vaccines (and/or low switched memory cells), and should have either increased susceptibility to infection, autoimmune manifestations, granulomatous disease, and/or unexplained polyclonal lymphoproliferation [2]. Up to 70% of CVID patients develop non-infectious inflammatory/immune-mediated complications, such as autoimmune cytopenia, interstitial lung disease (ILD), enteropathy, and liver disease [3]. CVID is a heterogeneous disorder where monogenic defects are found in ~ 10%, implying that several mechanisms of immune dysregulation can lead to these non-infectious complications [4]. It is unclear to which degree the individual clinical inflammatory complications have a shared pathogenesis, and various cells such as B cells, T cells, monocytes/macrophages, and type 3 innate lymphoid cells seem to be involved [5,6,7,8,9].
Approximately 20–30% of CVID patients develop ILD, which is associated with increased morbidity and mortality [10]. Pulmonary histological abnormalities in these patients reflect diversity and overlap, and include patterns of pulmonary lymphoid hyperplasia such as follicular bronchiolitis, lymphocytic interstitial pneumonitis, and nodular lymphoid hyperplasia as well as granulomas, organizing pneumonia, and fibrosis [10,11,12,13]. The term “granulomatous-lymphocytic interstitial lung disease” (GLILD) was proposed by Bates et al. to describe ILD in CVID with lymphocytic infiltrates and/or granulomas [10]. However, it is not clear if different entities of CVID-related ILD, also within the term GLILD, represent different pathological processes or variations within a disease spectrum. GLILD can often be distinguished radiologically from other ILDs by CT findings, and is characterized by reticulation, bronchial wall thickening, pulmonary nodules, and ground glass opacities [14,15,16].
The pathogenesis of GLILD is poorly understood. Small case series of immunohistochemistry performed on lung biopsies in GLILD have shown lymphocytic infiltrates with the presence of T cells and variable findings of B cell follicles within the infiltrates [11, 17, 18]. Maglione et al. found increased serum levels of B-cell activating factor (BAFF) in CVID patients with a progressive course of ILD compared to stable GLILD, suggesting that a BAFF-mediated resistance to apoptosis drives pulmonary B cell hyperplasia within the lungs [6, 18]. Friedmann et al. found that bronchoalveolar lavage (BAL) fluid of patients with CVID-related ILD had lower numbers of regulatory T cells, higher numbers of T follicular helper (TFH)-like memory cells skewed toward Th1 cells, and a larger fraction of B cells, mostly the inflammatory CD21low B cell subtype, as compared to patients with sarcoidosis [19].
In idiopathic pulmonary fibrosis and connective tissue disease related ILD, biomarkers such as surfactant protein D (SP-D), Club Cell protein 16 (CC16), and matrix metalloproteinase 7 (MMP-7) have been shown to work as diagnostic and prognostic biomarkers [20,21,22,23]. SP-D and CC16 appear to reflect airway epithelium injury, and MMP-7 is a marker of extracellular matrix (ECM) remodeling and fibrosis [24,25,26]. To the best of our knowledge, these markers, and other markers of ECM remodeling and growth factors with relevance for fibrogenesis, have not previously been examined in GLILD.
CVID patients with an inflammatory phenotype often develop several non-infectious complications. In this study, we aimed to identify biomarkers that could distinguish GLILD, or reflect the pathogenesis of GLILD distinctively, in a population of CVID patients where all have non-infectious complications. Accordingly, we designed this study by dividing the cohort of CVID patients with non-infectious complications in those with and those without GLILD, and kept CVID with infections only in a separate group. We analyzed selected soluble serum markers of inflammation, immune homeostasis, pulmonary epithelium injury, fibrogenesis, and ECM remodeling (see Table 1 for overview) based on previous studies of ILD, CVID, and various inflammatory disorders, and aimed to distinguish the serum profile of GLILD from other non-infectious complications in CVID. This could highlight novel aspects of GLILD pathogenesis and identify new therapeutic approaches and clinically useful biomarkers.
Materials and Methods
Ethics
The Regional Committee for Medical and Research Ethics approved the study protocol (REK no 2012/521 and 33,256). All study participants signed a written, informed consent. The work described has been carried out in accordance with the Declaration of Helsinki.
Patients and Subgroups
CVID patients were recruited from the Section of Clinical Immunology and Infectious Diseases, Oslo University Hospital, Rikshospitalet, Oslo, Norway. CVID was defined according to the European Society of Immunodeficiencies criteria [2]. The CVID patients were subdivided into three groups: “GLILD,” “other non-infectious complications” (OC), and “infections only” (IO). Patients with GLILD were diagnosed based on clinical and radiological features, and identified by review of medical records, including radiology reports. Chest high-resolution computed tomographies (HRCTs) of suspected GLILD patients were then reassessed, and the GLILD diagnosis was confirmed by two experienced chest radiologists in consensus [16]. The HRCT performed closest in time to the serum sampling was assessed quantitatively by a scoring tool described in a previous paper [16]. Patients were classified as progressive or stable as previously described [16, 18]. Non-infectious complications were defined according to Chapel’s classification of CVID phenotypes, including autoimmunity, polyclonal lymphocytic infiltration, and enteropathy [3]. We applied two modifications of the classification: (i) inclusion of biopsy-proven nodular regenerative hyperplasia and (ii) enteropathy defined as biopsy-proven lymphocytic infiltrations and/or persistent diarrhea after exclusion of gastrointestinal infection. Lung biopsies (trans-bronchial) were available from two GLILD-patients. All patients except two had been screened by a whole genome sequencing panel for primary immunodeficiencies.
Blood Sampling and Storage
The serum of patients was sampled between 2006 and 2013, collected in sterile tubes, allowed to coagulate at room temperature, centrifuged at 1500 g for 10 min, and stored at − 80 °C. Serum from healthy controls (HCs) was collected between 2010 and 2015 and stored likewise. None of the sample tubes had been thawed before. At the time of sampling, none of the patients had ongoing acute infection or immune modulating medical treatment (for corticosteroids, a daily dose ≤ 5 mg prednisolone was not defined as immune modulating).
Serum Analyses
Serum levels of Angiopoietin 2, BAFF, Cathepsin S, CC16, GDF-15, MMP-7, MMP-9, MPO, PARC, PAI-1, PECAM-1, periostin, TIMP-1, S100A8/A9, sBCMA, sCD14, sCD163, sCD25, SP-D, sTIM-3, VEGF, vWF, and YKL-40 were measured by enzyme immunoassays using commercially available antibodies (R&D Systems, Minneapolis, MN, except for vWF from Dako, Agilent, Santa Clara, CA) in a 384 format using a combination of a SELMA pipetting robot (Analytik Jena AG, Jena, Germany) and a BioTek dispenser/washer (BioTek Instruments, Winooski, Vt). Detection of eotaxin, IFN-γ, IL-6, IL-8, and TNF was performed by a MSD U-PLEX assay kit (Meso Scale Discovery, Rockville, MD). Intra- and inter-assay CVs were < 10%. See Table 1 for abbreviations.
B and T Cell Phenotyping
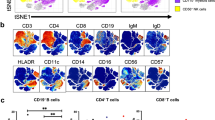
B and T cell counts and fractions of lymphocyte subsets were measured by flow cytometry in EDTA-anticoagulated blood sampled and analyzed within 14 months of the time point of serum sampling, at the Department of Immunology, Oslo University Hospital, Rikshospitalet. None of the patients received immune modulating medical treatment at the time of B/T cell phenotyping. Please see supplementary material for details, including Supplemental Figure S2.
Statistical Analyses
The primary aim of the study was to compare levels of serum markers in GLILD versus OC. Additionally, we wanted to compare the GLILD and the IO group. The CVID cohort as a whole was also compared to HCs in a separate analysis.
When examining the differences in biomarker levels between the three groups of CVID patients, our pre-defined statistical approach was to first compare these groups with the Kruskal–Wallis test for multiple group comparisons of continuous values. If the Kruskal–Wallis test yielded a p value < 0.05, differences between subject groups were assessed using Dunn’s multiple comparisons test, adjusted by Bonferroni for two comparisons (GLILD vs OC and GLILD vs IO). Mann–Whitney testing was used for two-group comparisons. Categorical values were compared using the chi-square test. Spearman rank correlation test was used for correlation analyses. Stepwise forward logistic regression analysis with GLILD as dependent factor was performed on log-transformed marker values as independent predictors. Calculations were performed in SPSS (version 26, IBM, NY). p-values < 0.05 were considered to be statistically significant.
Results
Patient Characteristics
The study cohort included 73 CVID patients. In total, 16 CVID patients (22%) were determined to have GLILD, 37 CVID patients (51%) had OC, and 20 CVID patients (27%) had IO (Fig. 1). These three CVID subgroups were similar in age, gender, BMI, Ig substitution form, and presence of bronchiectasis (Table 2). The distribution of complications other than GLILD was similar in the OC group and the GLILD group, except for a higher frequency of a previous history of autoimmune cytopenia in the GLILD group (38% in the GLILD group vs 8% in the OC group, p = 0.009). None of the patients had an ongoing episode of immune-driven cytopenia at the time of blood sampling.
The GLILD group had one patient with a CTLA4 haploinsufficiency, one with a STAT3 gain of function mutation and one with a variant of uncertain significance (VUS) in STAT3. In the OC group, there were three patients with likely pathogenic genetic variants (one heterozygote variant in NFKB1, one heterozygote deletion in IKZF1, and a de novo VUS in IRF2BP2). The IO group included three patients with likely pathogenic genetic variants (one with a heterozygote variant in CTLA4, one in IKZF1, and one in NFKB2).
Ten Markers Distinguish the CVID Subgroups: GLILD, OC, and IO
The three groups, GLILD, OC, and IO, were first tested by Kruskal–Wallis test for the 28 biomarkers given in Table 1. Ten markers were significantly different between the three groups: IFN-γ (p = 0.001), TNF (p = 0.007), sCD25 (p = 0.001) sTIM-3 (p = 0.001), sBCMA (p = 0.002), sCD163 (p = 0.044), SP-D (p = 0.007), CC16 (p = 0.034), MMP-7 (p = 0.020), and YKL-40 (p = 0.025) (Supplemental table S1). The levels of these ten markers were then, as predefined, selected for further comparisons between GLILD and OC, and also between GLILD and IO (Fig. 2).
Serum markers of ten biomarkers (a sCD25, b sTIM-3, c IFN-γ, d TNF, e SP-D, f CC16, g MMP-7, h sBCMA, i YKL-40 and j sCD163) associated with GLILD in a CVID population, selected by initial Kruskal Wallis testing. p values over the diagrams are calculated by Dunn’s multiple comparisons test (Bonferroni adjusted) for GLILD vs OC, and for GLILD vs IO. The IQRs of HCs are marked as shaded areas with dotted line at median
Increased Levels of T Cell Activation Markers in GLILD
Four of the ten selected markers could be related to T cell activation: sCD25, sTIM-3 (a marker of T cell activation and exhaustion), INF-γ, and TNF (both proposed markers of Th1 cell activation). We compared their levels in GLILD vs OC and GLILD vs IO, and found that the GLILD group had significantly higher levels of all four markers compared to OC as well as IO patients (Fig. 2a–d).
Elevated Pulmonary Epithelial Cell Injury and ECM Remodeling Markers in GLILD
Four of the selected markers could be related to pulmonary epithelial cell injury (SP-D and CC16) or ECM remodeling (MMP-7 and YKL-40), and were further explored between GLILD and OC, and GLILD and IO. We found significantly higher levels of SP-D, CC16, and MMP-7 in the GLILD group compared to the OC group (Fig. 2e–g). SP-D, MMP-7, and YKL-40 were higher in the GLILD group compared to the IO group, but not CC16 (Fig. 2e–g and i).
Higher Levels of sBCMA in GLILD
Serum levels of BAFF that has previously been suggested as a marker of GLILD progression did in the present study not come out significantly different in initial comparisons between the three CVID groups (Supplemental Table S1). In contrast, the levels of its soluble (s) receptor form, sBCMA, were significantly different between the three groups, and were compared between GLILD and OC, and GLILD and IO. We found markedly higher serum levels of sBCMA in the GLILD group compared to OC, but not compared to the IO group (Fig. 2h).
Higher Levels of sCD163 in GLILD Compared to IO
The monocyte/macrophage activation marker sCD163 was also differently regulated between the three groups, but we did not find a significant difference in serum levels of sCD163 between GLILD and OC, although the levels were significantly higher in the GLILD group compared to the IO group (Fig. 2j).
IFN-γ, sBCMA, and SP-D Were the Strongest Predictors of GLILD
We performed stepwise forward logistic regression of the eight biomarkers that were significantly higher in GLILD than OC, and found IFN-γ, sBCMA, and SP-D to be the strongest predictors of GLILD (p = 0.017, 0.016, and 0.039, respectively). GLILD patients had more frequently a history of autoimmune cytopenia. However, if we added AI cytopenia as a covariate in the logistic regression model, IFNγ, BCMA, and SP-D still come out as the strongest predictors of GLILD (data not shown).
Most Markers Upregulated in GLILD Were Stable Over Time
We had serial samples in 25 of the 73 CVID patients (eight from the GLILD group, 12 from the OC group, and five from the IO group), with a time interval between first and second sampling ranging from 0.5 to 6.4 years. None in the GLILD or the OC group had immune modulating treatment between the samplings that could have affected the results, and none of the GLILD patients had significant disease progression between the first and second sampling. The levels of sCD25, sTIM-3, INF-γ, TNF, SP-D, CC16, and MMP-7, which all were upregulated in GLILD, were stable over time (Supplemental Figure S1). In contrast, sBCMA had a significant decrease from the first to the second time point of sampling (Supplemental figure S1).
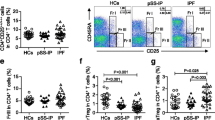
GLILD Patients Had Distinct Characteristics of Lymphocyte Subsets
Absolute counts of CD4+, CD8+, and CD19+ cells did not differ significantly between the three CVID subgroups (Table 2). The fraction of regulatory T cells (Treg) was however significantly lower in the GLILD group compared to both the OC and the IO group (p = 0.004 and p < 0.001 respectively), and the fraction of class-switched memory B cells was also lower in the GLILD group compared to OC and IO (p = 0.038 and p < 0.001 respectively). For CD21low B cells, the fraction in the GLILD group was significantly higher than in the OC group and in the IO group (p = 0.022 and 0.034 respectively). The fractions of follicular like CD4+ T cells were not different between the three groups (data not shown).
Levels of SP-D Were Higher in Patients with Progressive GLILD
Four of the 16 GLILD patients had progressive GLILD, as defined by pulmonary function tests over time, and the progressive patients had significantly higher levels of SP-D than those with stable GLILD (median of 0.899 and 0.144 arb. units respectively, p = 0.013). We did not find any significant difference in sCD25, sTIM-3, INFγ, TNF, MMP-7, CC16, or BCMA between patients with progressive and stable disease.
Levels of Serum Markers Did Not Correlate to Pulmonary CT Score
We could not find any significant correlations between the biomarkers specifically elevated in GLILD (sCD25, sTIM-3, IFNγ, TNF, SP-D, CC16, MMP-7, or sBCMA) and the total pulmonary CT pathology score, nor between the biomarkers and each radiological feature of GLILD.
Several Markers Distinguish CVID from Healthy Controls
Although detecting differences in serum markers between CVID patients and HCs was not the primary aim of this study, we also observed that CVID patients overall had significantly higher levels of Cathepsin S, S100A8A9, PARC, and GDF15, as well as significantly lower levels of MMP-9, periostin, Angiopoietin 2, and PAI-1 compared to 40 sex- and age-matched healthy controls (Supplemental Table S2).
Discussion
In the present study, we compared CVID patients with GLILD to CVID patients with other non-infectious complications to explore whether GLILD patients are characterized by a distinct profile of serum markers reflecting inflammation, pulmonary epithelial cell injury, ECM remodeling, and/or fibrogenesis. Our main findings were (i) GLILD patients had higher levels of sCD25, sTIM-3, TNF, and IFN-γ than CVID patients with OC, pointing to T cell activation and exhaustion as potentially central in GLILD pathogenesis. (ii) GLILD patients had higher levels of SP-D, CC16, and MMP-7; biomarkers of pulmonary epithelium injury; and ECM remodeling, compared to OC. (iii) Levels of BAFF were not significantly higher in our GLILD cohort, but its receptor, sBCMA, had increased levels in GLILD patients compared to the OC group. (iv) The significant markers reflecting T cell activation, airway epithelium injury, and ECM remodeling were shown to be consistent over time.
An increasing number of studies have highlighted the importance of T cell activation in the pathogenesis of the non-infectious complications of CVID [7, 8, 27, 28]. We have previously shown that sCD25 is increased in CVID compared to healthy controls, and also that sCD25 is significantly higher in CVID patients with non-infectious complications compared to infection only [29]. However, only a few studies have explored the role of T cell activation in GLILD. Berbers et al. found increased expression of Ki67 and IFN-γ in effector/memory CD4+ T cells as a sign of activation in patients with non-infectious complications compared to those with infections only, and pointed to more extreme findings in the GLILD patient subgroup [7]. Van Stigt et al. found elevated levels of sCD25 in a cohort of 12 patients with CVID and granulomatous disease (of which ten had GLILD/granulomas in lungs) compared to CVID patients with infection only [30]. In the present study, we extend these findings in several ways. By examining a larger CVID population than in previous reports, we found elevated levels of sCD25 in GLILD patients not only as compared to IO patients, but also compared to CVID patients with other non-infectious complications. sCD25 is released from the cell membrane of T cells as a result of activation, and has shown to be a useful biomarker in diseases such as sarcoidosis and hemophagocytic lymphohistiocytosis [31]. Our findings suggest that sCD25 could also be a relevant biomarker in GLILD.
Persistent T cell activation is often accompanied by T cell exhaustion, a dysfunctional state of activation, rendering the cell refractory to further stimuli. This can be reflected by upregulation of various check-point inhibitors like TIM-3. The soluble form of TIM-3 is a result of ADAM10-mediated shedding of its membrane-bound form, mainly on IFN-γ-producing CD4+ and CD8+ T cells [32]. Although the functional consequences of this shedding is not clear, levels of sTIM-3 have been shown to be a reliable marker of T cell activation/exhaustion in various disorders like progressive HIV, hepatitis virus C infection, and COVID-19 [32,33,34]. A few authors have suggested that T cell activation in CVID may be accompanied by exhaustion [8, 35], but the present study is the first to relate elevated sTIM-3 to CVID and GLILD. Although our finding of elevated sTIM-3 in GLILD is indicative of T cell exhaustion, parallel cellular studies should be performed in order to draw more firm conclusions. In relation to T cell homeostasis, we also found that fractions of Treg that we previously have reported lower in CVID patients with an inflammatory phenotype were lower in the GLILD group compared to the OC group [36]. This points to a distinctive immune dysregulation in GLILD.
In line with the role of IFN-γ-producing CD4+ and CD8+ T cells as cellular sources of sTIM-3, we found elevated levels of the Th1-derived cytokine IFN-γ in GLILD patients compared to the other CVID groups. Although TNF have several cellular sources, Th1 cells are important contributors, and the raised TNF levels in GLILD patients further underscore the role of Th1 cells in GLILD. To summarize, higher levels of sCD25 and sTIM-3 combined with the Th1 response signature cytokines TNF and IFN- γ in GLILD are consistent findings suggesting that activated T cells play an important role in GLILD pathogenesis.
In the present study, we have showed that GLILD patients have higher levels of CC16 (an anti-inflammatory protein secreted by non-ciliated respiratory epithelium), SP-D (a neutralizing, anti-viral lipoprotein complex secreted by type II alveolar cells and bronchiolar epithelium [25, 37]), and MMP-7 (a protease expressed by epithelial cells, fibroblasts, and macrophages that activates microbicidal α-defensins [24]) than OC patients. In ILDs, elevated levels of CC16 and SP-D may reflect airway epithelial injury [21, 26]. Levels of CC16 are demonstrated to correlate with disease activity of ILD in systemic sclerosis, and SP-D has shown to be useful to diagnose and predict risk of exacerbation and mortality in idiopathic pulmonary fibrosis [38, 39]. SP-D in combination with MMP-7 can facilitate the identification of rheumatoid arthritis associated ILD at an early stage [40]. In GLILD, more than half of the patients have a progressive phenotype, with declining DLCO and FVC over time [16, 18]. This study suggests that CC16, SP-D, and MMP-7 could serve as biomarkers in GLILD even if their prognostic value is currently uncertain. In fact, SP-D came out as one of the strongest predictors of GLILD by stepwise regression. In line with previous reports, we found that GLILD patients had higher percentage of CD21low and lower percentage of class-switched memory B cells [41, 42]. However, unlike Maglione et al., we were not able to demonstrate significantly higher levels of BAFF in the GLILD group. Instead, we did find higher levels of sBCMA in the GLILD group compared to the OC group. BCMA is shedded as sBCMA from plasma cells, and regulates plasma cell proliferation in bone marrow [43]. CVID patients overall are known to have substantially lower levels of sBCMA than healthy subjects, as confirmed in our cohort, reflecting the maturation defect of the B-cell lineage [44]. Although BCMA is mainly expressed on plasma cells, we cannot say if these or other cells are the source of sBCMA in GLILD. This issue may be subject for further studies, preferentially of bone marrow and lung biopsies of GLILD patients.
Currently, there is no consensus regarding optimal treatment of GLILD; however, rituximab combined with azathioprine or mycophenolate is often used and has shown effect in retrospective studies [6, 45]. Our results suggest that the use of treatment targeting T cells should be further explored. Although rituximab directly targets CD20+ cells, it also limits T cell activation by impairing B cell antigen presentation. Data on more T cell-specific therapy for GLILD are scarce. However, in a pilot study of treatment with the CTLA-4 Ig fusion protein abatacept, Warnatz et al. reported favorable results in five of eight CVID patients with ILD [46], and a case report of two GLILD patients describes successful treatment with the mTOR inhibitor sirolimus [47]. Inhibitors of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway suppress intracellular signaling mediated by multiple cytokines, also afflicting T cells directly [48]. JAK inhibitors have been used to treat inflammatory conditions of some inborn errors of immunity: STAT3 and STAT1 gain-of-function mutations and type I interferonopathies, but there are as far as we know no reports of their use in CVID or GLILD.
The main strength of this study is a well-characterized CVID cohort with a substantial number of patients in all three subgroups, GLILD, OC, and IO. Another strength is that we used a simple and predefined statistical design with handpicked relevant serum markers for CVID and ILD/GLILD rather than using fixed multiplex panels. One limitation is that even though we compared GLILD patients to a CVID group of other non-infectious complications, we cannot rule out that other differences between the groups could have affected the results.
Studies in larger GLILD cohorts could clarify if the biomarkers we identified correlate with pulmonary function, treatment effect, and prognosis. The retrospective design is an important limitation of the study, as is the lack of data from bronchial lavage fluid and pulmonary biopsies from GLILD patients.
Conclusions
Our findings suggest that T cell activation and exhaustion, pulmonary epithelium injury, and ECM remodeling are central and distinct features of GLILD pathogenesis potentially reflecting novel targets for therapy and promising biomarkers for clinical use. However, there is a need for larger prospective studies that also include pulmonary biopsy material.
Data Availability
The datasets analyzed during the current study are not publicly available due to Norwegian legislation regarding general data protection regulation but are available from the corresponding author (MSAF), on reasonable request.
References
Cunningham-Rundles C. How I treat common variable immune deficiency. Blood. 2010;116(1):7–15.
Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019;7(6):1763–70.
Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86.
de Valles-Ibáñez G, Esteve-Solé A, Piquer M, González-Navarro EA, Hernandez-Rodriguez J, Laayouni H, et al. Evaluating the genetics of common variable immunodeficiency: monogenetic model and beyond. 2018;9.
Maglione PJ, Cols M, Cunningham-Rundles C. Dysregulation of innate lymphoid cells in common variable immunodeficiency. Curr Allergy Asthma Rep. 2017;17(11):77.
Maglione PJ, Gyimesi G, Cols M, Radigan L, Ko HM, Weinberger T, et al. BAFF-driven B cell hyperplasia underlies lung disease in common variable immunodeficiency. JCI Insight. 2019;4(5).
Berbers RM, van der Wal MM, van Montfrans JM, Ellerbroek PM, Dalm V, van Hagen PM, et al. Chronically activated T-cells retain their inflammatory properties in common variable immunodeficiency. J Clin Immunol. 2021;41(7):1621–32.
Berbers RM, Drylewicz J, Ellerbroek PM, van Montfrans JM, Dalm V, van Hagen PM, et al. Targeted proteomics reveals inflammatory pathways that classify immune dysregulation in common variable immunodeficiency. J Clin Immunol. 2021;41(2):362–73.
Abyazi ML, Bell KA, Gyimesi G, Baker TS, Byun M, Ko HM, et al. Convergence of cytokine dysregulation and antibody deficiency in common variable immunodeficiency with inflammatory complications. J Allergy Clin Immunol. 2022;149(1):315-26.e9.
Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114(2):415–21.
Rao N, Mackinnon AC, Routes JM. Granulomatous and lymphocytic interstitial lung disease: a spectrum of pulmonary histopathologic lesions in common variable immunodeficiency–histologic and immunohistochemical analyses of 16 cases. Hum Pathol. 2015;46(9):1306–14.
Dhalla F, Lochlainn DJM, Chapel H, Patel SY. Histology of interstitial lung disease in common variable immune deficiency. Front Immunol. 2020;11: 605187.
Larsen BT, Smith ML, Tazelaar HD, Yi ES, Ryu JH, Churg A. GLILD revisited: pulmonary pathology of common variable and selective IgA immunodeficiency. Am J Surg Pathol. 2020;44(8):1073–81.
Cabanero-Navalon MD, Garcia-Bustos V, Forero-Naranjo LF, Baettig-Arriagada EJ, Núñez-Beltrán M, Cañada-Martínez AJ, et al. Integrating clinics, laboratory, and imaging for the diagnosis of common variable immunodeficiency-related granulomatous-lymphocytic interstitial lung disease. Front Immunol. 2022;13: 813491.
Meerburg JJ, Hartmann IJC, Goldacker S, Baumann U, Uhlmann A, Andrinopoulou E-R, et al. Analysis of granulomatous lymphocytic interstitial lung disease using two scoring systems for computed tomography scans—a retrospective cohort study. 2020;11.
Fraz MSA, Moe N, Revheim M-E, Stavrinou ML, Durheim MT, Nordøy I, et al. Granulomatous-lymphocytic interstitial lung disease in common variable immunodeficiency—features of CT and 18F-FDG positron emission tomography/CT in Clinically Progressive Disease. 2021;11.
Patel S, Anzilotti C, Lucas M, Moore N, Chapel H. Interstitial lung disease in patients with common variable immunodeficiency disorders: several different pathologies? Clin Exp Immunol. 2019;198(2):212–23.
Maglione PJ, Overbey JR, Cunningham-Rundles C. Progression of common variable immunodeficiency interstitial lung disease accompanies distinct pulmonary and laboratory findings. J Allergy Clin Immunol Pract. 2015;3(6):941–50.
Friedmann D, Unger S, Keller B, Rakhmanov M, Goldacker S, Zissel G, et al. Bronchoalveolar lavage fluid reflects a TH1-CD21 low B-cell interaction in CVID-related interstitial lung disease. 2021;11.
White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016;194(10):1242–51.
Buendía-Roldán I, Ruiz V, Sierra P, Montes E, Ramírez R, Vega A, et al. Increased expression of CC16 in patients with idiopathic pulmonary fibrosis. PLoS ONE. 2016;11(12): e0168552.
Janssen R, Sato H, Grutters JC, Bernard A, van Velzen-Blad H, du Bois RM, et al. Study of Clara cell 16, KL-6, and surfactant protein-D in serum as disease markers in pulmonary sarcoidosis. Chest. 2003;124(6):2119–25.
Inoue Y, Kaner RJ, Guiot J, Maher TM, Tomassetti S, Moiseev S, et al. Diagnostic and prognostic biomarkers for chronic fibrosing interstitial lung diseases with a progressive phenotype. Chest. 2020;158(2):646–59.
Burke B. The role of matrix metalloproteinase 7 in innate immunity. Immunobiology. 2004;209(1–2):51–6.
Zhai J, Insel M, Addison KJ, Stern DA, Pederson W, Dy A, et al. Club cell secretory protein deficiency leads to altered lung function. Am J Respir Crit Care Med. 2019;199(3):302–12.
Sorensen GL. Surfactant protein D in respiratory and non-respiratory diseases. Front Med. 2018;5:18.
Hultberg J, Ernerudh J, Larsson M, Nilsdotter-Augustinsson Å, Nyström S. Plasma protein profiling reflects T(H)1-driven immune dysregulation in common variable immunodeficiency. J Allergy Clin Immunol. 2020;146(2):417–28.
Unger S, Seidl M, van Schouwenburg P, Rakhmanov M, Bulashevska A, Frede N, et al. The T(H)1 phenotype of follicular helper T cells indicates an IFN-γ-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J Allergy Clin Immunol. 2018;141(2):730–40.
Jørgensen SF, Trøseid M, Kummen M, Anmarkrud JA, Michelsen AE, Osnes LT, et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol. 2016;9(6):1455–65.
van Stigt AC, Dalm VASH, Nagtzaam NMA, van Rijswijk DA, Barendregt BH, van Hagen PM, et al. Soluble interleukin-2 receptor is a promising serum biomarker for granulomatous disease in common variable immune deficiency. J Clin Immunol. 2021;41(3):694–7.
Damoiseaux J. The IL-2 - IL-2 receptor pathway in health and disease: the role of the soluble IL-2 receptor. Clin Immunol (Orlando, FL). 2020;218: 108515.
Clayton KL, Douglas-Vail MB, Nur-ur Rahman AK, Medcalf KE, Xie IY, Chew GM, et al. Soluble T cell immunoglobulin mucin domain 3 is shed from CD8+ T cells by the sheddase ADAM10, is increased in plasma during untreated HIV infection, and correlates with HIV disease progression. J Virol. 2015;89(7):3723–36.
Hoel H, Ueland T, Hove-Skovsgaard M, Hartling HJ, Gelpi M, Benfield T, et al. Soluble T-cell immunoglobulin mucin domain-3 is associated with gepatitis C virus coinfection and low-grade inflammation during chronic human immunodeficiency virus infection. Open Forum Infect Dis. 2020;7(2):ofaa033.
Ueland T, Heggelund L, Lind A, Holten AR, Tonby K, Michelsen AE, et al. Elevated plasma sTIM-3 levels in patients with severe COVID-19. J Allergy Clin Immunol. 2021;147(1):92–8.
Klocperk A, Unger S, Friedmann D, Seidl M, Zoldan K, Pfeiffer J, et al. Exhausted phenotype of follicular CD8 T cells in CVID. J Allergy Clin Immunol. 2020;146(4):912-5.e13.
Fevang B, Yndestad A, Sandberg WJ, Holm AM, Müller F, Aukrust P, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007;147(3):521–5.
Watson A, Madsen J, Clark HW. SP-A and SP-D: Dual functioning immune molecules with antiviral and immunomodulatory Properties. 2021;11.
Wang K, Ju Q, Cao J, Tang W, Zhang J. Impact of serum SP-A and SP-D levels on comparison and prognosis of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Medicine. 2017;96(23): e7083.
Hasegawa M, Fujimoto M, Hamaguchi Y, Matsushita T, Inoue K, Sato S, et al. Use of serum clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J Rheumatol. 2011;38(5):877–84.
Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015;191(12):1403–12.
Hartono S, Motosue MS, Khan S, Rodriguez V, Iyer VN, Divekar R, et al. Predictors of granulomatous lymphocytic interstitial lung disease in common variable immunodeficiency. Ann Allergy Asthma Immunol. 2017;118(5):614–20.
Cinetto F, Scarpa R, Carrabba M, Firinu D, Lougaris V, Buso H, et al. Granulomatous lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID): a multicenter retrospective study of patients from Italian PID referral centers. Front Immunol. 2021;12: 627423.
Laurent SA, Hoffmann FS, Kuhn P-H, Cheng Q, Chu Y, Schmidt-Supprian M, et al. γ-secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6(1):7333.
Maglione PJ, Ko HM, Tokuyama M, Gyimesi G, Soof C, Li M, et al. Serum B-cell maturation antigen (BCMA) levels differentiate primary antibody deficiencies. J Allergy Clin Immunol Pract. 2020;8(1):283-91.e1.
Verbsky JW, Hintermeyer MK, Simpson PM, Feng M, Barbeau J, Rao N, et al. Rituximab and antimetabolite treatment of granulomatous and lymphocytic interstitial lung disease in common variable immunodeficiency. J Allergy Clin Immunol. 2021;147(2):704-12.e17.
von Spee-Mayer C, Echternach C, Agarwal P, Gutenberger S, Soetedjo V, Goldacker S, et al. Abatacept use is associated with steroid dose reduction and improvement in fatigue and CD4-dysregulation in CVID patients with interstitial lung disease. J Allergy Clin Immunol Pract. 2021;9(2):760-70.e10.
Deyà-Martínez A, Esteve-Solé A, Vélez-Tirado N, Celis V, Costa J, Cols M, et al. Sirolimus as an alternative treatment in patients with granulomatous-lymphocytic lung disease and humoral immunodeficiency with impaired regulatory T cells. Pediatr Allergy Immunol. 2018;29(4):425–32.
Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct Target Ther. 2021;6(1):402.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital) SFJ and MEM were funded by grants from the South-Eastern Norway Regional Health Authority (project numbers 2019089 and 2012/521, respectively).
Author information
Authors and Affiliations
Contributions
MSAF, AEM, TU, SFJ, PA, and BF contributed to the study conception. MSAF, MEM, BF, SFJ, IN, AMH, and PA collected clinical data. AEM and ET performed analyses. MSAF and TU performed statistical analyses. NM and TMA reviewed CT images. MSAF and BF drafted the paper, and all authors critically revised the manuscript for important intellectual content and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol is approved by the Regional Committee for Medical and Research Ethics, South East Norway (protocol nos. 2012/521 and 33256).
Consent to Participate and to Publish
Written informed consent to participate in the study was obtained from all participants. All patients signed informed consent regarding publishing their data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fraz, M.S.A., Michelsen, A.E., Moe, N. et al. Raised Serum Markers of T Cell Activation and Exhaustion in Granulomatous-Lymphocytic Interstitial Lung Disease in Common Variable Immunodeficiency. J Clin Immunol 42, 1553–1563 (2022). https://doi.org/10.1007/s10875-022-01318-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01318-1