Abstract
Introduction
Inflammatory bowel disease (IBD) affects approximately 1/3 of patients with chronic granulomatous disease (CGD). Comprehensive investigation of the effect of allogeneic hematopoietic cell transplantation (HCT) on CGD IBD and the impact of IBD on transplant outcomes is lacking.
Methods
We collected data retrospectively from 145 patients with CGD who had received allogeneic HCT at 26 Primary Immune Deficiency Treatment Consortium (PIDTC) centers between January 1, 2005 and June 30, 2016.
Results
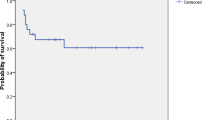
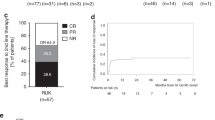
Forty-nine CGD patients with IBD and 96 patients without IBD underwent allogeneic HCT. Eighty-nine percent of patients with IBD and 93% of patients without IBD engrafted (p = 0.476). Upper gastrointestinal acute GVHD occurred in 8.5% of patients with IBD and 3.5% of patients without IBD (p = 0.246). Lower gastrointestinal acute GVHD occurred in 10.6% of patients with IBD and 11.8% of patients without IBD (p = 0.845). The cumulative incidence of acute GVHD grades II–IV was 30% (CI 17–43%) in patients with IBD and 20% (CI 12–29%) in patients without IBD (p = 0.09). Five-year overall survival was equivalent for patients with and without IBD: 80% [CI 66–89%] and 83% [CI 72–90%], respectively (p = 0.689). All 33 surviving evaluable patients with a history of IBD experienced resolution of IBD by 2 years following allogeneic HCT.
Conclusions
In this cohort, allogeneic HCT was curative for CGD-associated IBD. IBD should not contraindicate HCT, as it does not lead to an increased risk of mortality.
This study is registered at clinicaltrials.gov NCT02082353.





Similar content being viewed by others
Change history
29 August 2020
The original version of this article unfortunately contained the missing author, Caridad Martinez. The authors would like to correct the list. We apologize for any inconvenience that this may have caused. The correct author list is shown above.
References
Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O'Brien S, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114(2):462–8.
Jones LB, McGrogan P, Flood TJ, et al. Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin Exp Immunol. 2008;152(2):211–8.
Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn's disease. Am J Gastroenterol. 2009;104(1):117–24.
Khangura SK, Kamal N, Ho N, Quezado M, Zhao X, Marciano B, et al. Gastrointestinal features of chronic granulomatous disease found during endoscopy. Clin Gastroenterol Hepatol. 2016;14(3):395–402 e395.
Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis. 2010;51(12):1429–34.
Campbell N, Chapdelaine H. Treatment of chronic granulomatous disease-associated fistulising colitis with vedolizumab. J Allergy Clin Immunol Pract. 2017;5(6):1748–9.
de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A. 2014;111(9):3526–31.
van de Veerdonk FL, Netea MG, Dinarello CA, van der Meer JW. Anakinra for the inflammatory complications of chronic granulomatous disease. Neth J Med. 2011;69(2):95.
Hahn KJ, Ho N, Yockey L, Kreuzberg S, Daub J, Rump A, et al. Treatment with Anakinra, a recombinant IL-1 receptor antagonist, unlikely to induce lasting remission in patients with CGD colitis. Am J Gastroenterol. 2015;110(6):938–9.
Angelino G, De Angelis P, Faraci S, et al. Inflammatory bowel disease in chronic granulomatous disease: an emerging problem over a twenty years' experience. Pediatr Allergy Immunol. 2017;28(8):801–9.
Soncini E, Slatter MA, Jones LB, et al. Unrelated donor and HLA-identical sibling haematopoietic stem cell transplantation cure chronic granulomatous disease with good long-term outcome and growth. Br J Haematol. 2009;145(1):73–83.
Seger RA, Gungor T, Belohradsky BH, Blanche S, Bordigoni P, di Bartolomeo P, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985-2000. Blood. 2002;100(13):4344–50.
Gungor T, Teira P, Slatter M, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383(9915):436–48.
Khandelwal P, Bleesing JJ, Davies SM, Marsh RA. A single-center experience comparing Alemtuzumab, Fludarabine, and Melphalan reduced-intensity conditioning with Myeloablative Busulfan, cyclophosphamide, and antithymocyte globulin for chronic granulomatous disease. Biol Blood Marrow Transplant. 2016;22(11):2011–8.
Parta M, Kelly C, Kwatemaa N, Theobald N, Hilligoss D, Qin J, et al. Allogeneic reduced-intensity hematopoietic stem cell transplantation for chronic granulomatous disease: a single-center prospective trial. J Clin Immunol. 2017;37(6):548–58.
Morillo-Gutierrez B, Beier R, Rao K, Burroughs L, Schulz A, Ewins AM, et al. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: a multicenter experience. Blood. 2016;128(3):440–8.
Marsh R, Hebert KM, Keesler D, et al. Practice pattern changes and improvements in hematopoietic cell transplantation for primary Immunodeficiencies. J Allergy Clin Immunol. 2018;142:2004–7.
Hauck F, Koletzko S, Walz C, Bernuth H, Klenk A, Schmid I, et al. Diagnostic and treatment options for severe IBD in female X-CGD carriers with non-random X-inactivation. J Crohns Colitis. 2016;10(1):112–5.
Griffith LM, Cowan MJ, Notarangelo LD, Kohn DB, Puck JM, Shearer WT, et al. Primary immune deficiency treatment consortium (PIDTC) update. J Allergy Clin Immunol. 2016;138(2):375–85.
Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363(27):2600–10.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33.
Marciano BE, Zerbe CS, Falcone EL, Ding L, DeRavin SS, Daub J, et al. X-linked carriers of chronic granulomatous disease: illness, lyonization, and stability. J Allergy Clin Immunol. 2018;141(1):365–71.
Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17.
Seger RA. Hematopoietic stem cell transplantation for chronic granulomatous disease. Immunol Allergy Clin N Am. 2010;30(2):195–208.
Battersby AC, Braggins H, Pearce MS, Cale CM, Burns SO, Hackett S, et al. Inflammatory and autoimmune manifestations in X-linked carriers of chronic granulomatous disease in the United Kingdom. J Allergy Clin Immunol. 2017;140(2):628–630 e626.
Acknowledgements
We thank Elizabeth Dunn and Tara Bani-Hashemi for project management and assistance. We thank all of the study coordinators for collection of clinical data from PIDTC sites. We thank all of the clinical care teams who provided care for patients.
Funding
The PIDTC is supported by the Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases (NIAID); and the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), Bethesda, MD; Public Health Service grant/cooperative agreements U54-AI082973 (PI: MJ Cowan), U54-NS064808 and U01-TR001263 (PI: JP Krischer) and R13-AI094943 (PI: MJ Cowan).
The PIDTC is a part of the Rare Diseases Clinical Research Network (RDCRN) of ORDR, NCATS. The content and opinions expressed are solely the responsibility of the authors and do not represent the official policy or position of the NIAID, ORDR, NCATS, NIH, or any other agency of the US Government.
Support was also provided by the Division of Intramural Research, NIAID, NIH (EMK, LDN, HLM, PG and DG).
Author information
Authors and Affiliations
Consortia
Contributions
The eligibility review expert panel (MJC, DBK, EMK, RAM, LMG, SP, JMP, LDN, EH, JWL, ES, MAP) reviewed each submitted case to assess eligibility criteria. The study was designed by EMK, HLM, LDN, and LMG. MJC, JMP, EH, DBK, LMG, LDN, and SYP served as senior leadership on the PIDTC Steering Committee. RAM, JWL, HLM, SP, and EMK wrote the first draft of the manuscript. BRL, ZY, and KP performed statistical analyses. RAM, JWL, DA, EH, ELF, EA, JJB, KES, JH, LMB, SSS, SC, LY, BRO, GC, MT, KC, PT, SS, RP, LF, DC, BJDS, AJS, KW, AJ, FB, TQ, CCD, DG, TT, PG, VP, AK, HC, HM, MTLM, KDS, MJC, LDN, DBK, ES, SYP, JMR, JMP, NK, MAP, HLM, SP, and EMK contributed patients and/or provided clinical care and/or entered patient data. All authors edited the manuscript and contributed to the subsequent versions.
LMG, an employee of DAIT, NIAID, the funding source, also participated as a PIDTC study team member in the interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• CGD-associated inflammatory bowel disease (IBD) resolves after allogeneic hematopoietic cell transplant (HCT).
• Five-year survival following allogeneic HCT was greater than 80% in this PIDTC CGD cohort, and baseline IBD did not adversely affect survival.
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Marsh, R.A., Leiding, J.W., Logan, B.R. et al. Chronic Granulomatous Disease-Associated IBD Resolves and Does Not Adversely Impact Survival Following Allogeneic HCT. J Clin Immunol 39, 653–667 (2019). https://doi.org/10.1007/s10875-019-00659-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-019-00659-8