Abstract
Objective
This study aimed to determine whether Mycobacterium bovis Bacillus Calmette-Guérin (BCG) treatment can reverse an established allergic airway inflammation in a BALB/c mouse model of ovalbumin (OVA)-induced airway inflammation.
Methods
OVA sensitized BALB/c mice were challenged with aerosolized OVA on days 28 to 30, 34, 41 and 63. Mice were intranasal treated with BCG on days 35 and 42. Twenty-four hours after the last challenge, blood samples were collected to detect anti-OVA immunoglobulin isotypes, and bronchoalveolar lavage (BAL) was harvested for cell count. Additionally, lungs were collected for histological analysis, detection of the eosinophil peroxidase (EPO) activity and measurement of cytokines and CCL11. The expression of CTLA-4, Foxp3 and IL-10 was also determined in lung tissue by flow cytometry.
Results
BCG treatment was able to inhibit an established allergic Th2-response, by decreasing the allergen-induced eosinophilic inflammation, EPO activity, levels of CCL11 and IL-4, serum levels of IgE and IgG1. Mycobacteria treatment increased lung levels of IFN-γ, IL-10 and TGF-β, and expressions of Foxp3 and CTLA-4 in CD4+T cells. Additionally, an increased production of IL-10 by CD8+ T cells was observed, even though no detectable changes in CD4+IL-10+ was noticed.
Conclusion
BCG treatment inhibits features of allergic airway inflammation and the results suggest that the mechanism underlying the down-regulatory effects of BCG on OVA-induced airway inflammation appear to be associated with the induction of both Th1 and T regulatory immune responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic asthma is a chronic respiratory disease characterized by airway hyperresponsiveness, reversible airway obstruction, lung inflammation, and high levels of allergen-specific IgE [1]. T helper 2 cells are thought to play a pivotal role in development of allergic response by the cytokines that they secrete, such as IL-4, IL-5, IL-9 and IL-13, and chemokines, including CCL11, which play an important role in inflammatory cell recruitment [2]. These mediators contribute to the clinical features of allergic pathogeneses by triggering IgE production, eosinophil inflammation, mucus hypersecretion and bronchial hyperreactivity [3].
The increasing prevalence of allergic diseases and asthma, particularly in industrialized countries, has led to the hygiene hypothesis, which states that the newborn infant’s immune system is skewed toward Th2 responses and needs timely and appropriate environmental stimulus to create a balanced immune response [4, 5]. Supporting this hypothesis, epidemiological and experimental evidence has shown an inverse correlation between Th1-induced microbial infections and allergic diseases [6]. Similarly, some animal studies have demonstrated that exposure to Mycobacterium tuberculosis or to environmental mycobacteria is able to protect against the development of allergic responses [7, 8], introducing the concept that the administration of mycobacteria and their products may therefore be used as vaccines aimed at inhibiting Th2 responses. However the exact mechanism underlying this inhibition still remains poorly understood. Induction of Th1 cytokines, such as interferon-γ, has been suggested to cause a shift from Th2 to Th1 immune responses [9, 10], thus subsequently preventing inflammatory allergic immune response. Nevertheless some results indicated that the immunological mechanism behind this inhibition it is much more complex than is predicted by this and additional non-Th1-Th2 immunological regulatory mechanism may play a much more prominent role [11]. In this context, some clinical and experimental studies have also highlighted the important role of regulatory T cells (Treg) in the control of allergic diseases, providing evidence that it works in a manner that is, in part, dependent on IL-10 or TGF-β or both [12–14]. Additionally, it has been well established that the transcription factor Foxp3, as well as the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), has a key role in the function of Treg cells [15, 16] and would be crucial to modulate an allergen-induced inflammation [17]. Furthermore, it has been demonstrated that treatment of newborn mice with Mycobacterium bovis—BCG or Mycobacterium vaccae before ovalbumin-sensitization was effective in reducing allergen induced-airway eosinophilia by giving rise to Treg cells [18]. On the other hand, some studies failed to show an effective suppression of allergic features after an allergen challenge [19], suggesting that the therapeutic efficacy of BCG treatment in mouse models of allergy remains controversial. The present study aimed to evaluate the ability of BCG to suppress an established allergic response in a BALB/c mouse model of OVA-induced airway inflammation to achieve new insights into the mechanisms of how mycobacteria exert their anti-inflammatory effect.
Methods
Animals
Female BALB/c mice 6 to 8 weeks old were obtained from the Universidade Federal de Juiz de Fora (UFJF) animal care facility and were housed in microisolator cages receiving chow and water ad libitum. Animals were grouped according to treatment as follows (n = 5): (a) PBS (sensitizations and airway challenges with phosphate-buffered saline); (b) BCG (sensitizations and airway challenges with PBS plus BCG treatment); (c) OVA (sensitizations and airway challenges with OVA); (d) OVA/BCG (sensitizations and airway challenges with OVA plus BCG treatment). All animal experimentation was in accordance with the principles of Brazilian Code for the Use of Laboratory Animals and was approved by UFJF Ethics Committee on the use of laboratory animals (CEEA–UFJF No. 027/2008).
Ovalbumin-Induced Airway Inflammation and BCG Treatment
Mice were sensitized intraperitoneally (i.p.) on days 0 and 14 with 3 μg of OVA (Grade V, Sigma-Aldrich Corp., USA) in 1 mg of alum (Sigma-Aldrich Corp., USA). Animals were challenged for 20 min with aerosolized OVA 1 % or PBS (Inalatec Plus, Nevoni, Brazil) on days 28 to 30, 34, 41 and 63. Mice were twice intranasal treated on days 35 and 42 with 50 μL of 2 × 106 CFU/mL of Mycobacterium bovis bacillus Calmette-Guérin (Moreau substrain, Ataupho de Paiva Foundation, Rio de Janeiro, Brazil) or PBS (Fig. 1).
Collection of Blood, Bronchoalveolar Lavage and Lung Samples
Twenty-four hours after the last OVA challenge mice were anesthetized i.p. with a solution of ketamine (90 mg/kg, Syntec, Brazil) and xylazine (10 mg/kg, Calmiun, Agener União, Brazil) and exsanguinated via the brachial plexus. The blood samples were centrifuged at 7,500 x g for 2 min (Centrifuge 5410, Eppendorf, Germany), and the serum was separated and stored at -20 °C. Subsequently, bronchoalveolar lavage (BAL) was performed by intratracheal instillation of 1 mL of PBS three times and cell pellets were resuspended in 1 mL of PBS containing 2 % fetal bovine serum. Then the chest cavity was opened to remove the lung lobes.
Total and Specific Cell Counts in BAL
Total leukocytes count was determined and percentages of a differential count of 300 leukocytes in BAL were determined using standard morphological criteria, examining cytospin slides (Fanem 248, Brazil) by Panoptic staining (Laborclin Ltda, Pinhais, Brazil).
Eosinophil Peroxidase Activity
For determination of eosinophil peroxidase (EPO) activity 100 mg of lung tissue was homogenized in 1.9 mL PBS, and centrifuged at 12,000 x g (Jouan, Thermo Electron Corporation, USA) for 10 min at 4 °C. The pellet was suspended in 1.9 mL of 0.5 % HTAB in PBS. The samples were frozen three times in liquid nitrogen and centrifuged at 10,000 x g for 10 min at 4 °C (Thermo Electron CR312, USA). The supernatant was used in the enzymatic assay. Briefly, 1.5 mL of o-phenyldiamine (OPD) solution was added to 8.5 mL of tris buffer, followed by the addition of 7.5 μL of H2O2. Using a 96-well plate 100 μL of substrate solution was added to 50 μL of each sample. After 30 min, the reaction was stopped with 50 μL of H2SO4 1 M and the absorbance measured at 492 nm in a microplate reader (SPECTRAMAX 190, Molecular Devices, USA).
Measurement of Cytokines and Chemokine Levels in Lungs
In order to evaluate the levels of cytokines and chemokine in lung tissues, 100 mg of tissue was homogenized in 1 mL of PBS containing protease inhibitors (0.1 mM phenylmethylsulphonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA and 2 μL of aprotinin A) and 0.05 % Tween 20. Samples were then centrifuged for 15 min at 500 x g (Thermo Electron CR312, USA) and the levels of cytokines (IL-4, IL-5, IL-13, IFN-γ, TGF-β and IL-10) and the chemokine CCL11 in supernatants were measured using commercially available ELISA kits (OptEIA, BD Bioscience for cytokines and R&D Diagnostics for chemokines), in accordance with the manufacturer’s instructions.
Measurement of Anti-OVA-Specific IgE, IgG1 and IgG2a Antibodies
Quantification of anti-OVA-specific IgE, IgG1 and IgG2a antibodies was determined by ELISA. Briefly, 96-wells plates were sensitized with ovalbumin (10 μg/mL) and incubated for 18 h at 4 °C. The plates were washed with 0.05 % Tween 20-PBS (PBST) and blocked with 10 % fetal bovine serum-PBST for 2 h at room temperature. The plates were washed with PBST and 50 μL of each serum (1:20 for IgE, and 1:100 dilutions for IgG1 and IgG2a) was added per well, and incubated for 18 h at 4 °C. The plates were then washed and biotin-conjugated rat anti-mouse IgE, IgG1 or IgG2a (1:1,000; Pharmingen, BD Bioscience) was added. After 1 h of incubation at room temperature a substrate solution containing 0.1 M citric acid, 0.2 M sodium phosphate, distilled water, OPD, and 0.03 % hydrogen peroxide was added. The reaction was stopped with H2SO4 1 M and the optical density measured at 492 nm in a microplate reader (Spectramax 190; Molecular Devices).
Cell Staining and Flow Cytometry
The lungs were removed and mashed through a 70 μm cell strainer. The red blood cells were lysed with ACK buffer and the cell suspension was washed twice in RPMI 1640 and adjusted to 1 × 106 cells per well. Cells were then stained with florescence-conjugated antibodies for cell surface markers, including PerCP-anti-CD4, PerCP-anti-CD8 and PE-anti-CD152 (all BD Bioscience—Pharmingen). For Foxp3 and IL-10 expression measurement, the cells were collected and stained for cell surface markers (CD4, CD8) without stimulation as described by the manufacturer's instructions. After surface marker staining, cells were permeabilized and stained with PE-anti-IL-10 and Alexa Fluor 488-anti-Foxp3 (BD Bioscience—Pharmingen). Data acquisition was performed using FACSCalibur (Becton Dickinson, San Jose, CA). Data analysis was performed using FCS Express software (De Novo).
Lung Pathology
The right upper lung lobe of each animal was fixed in 10 % buffered formalin and the samples were submitted to routine histologic processing. Tissue sections were stained with hematoxylin and eosin (HE), and periodic acid-Schiff (PAS) and examined in blinded fashion on an optical microscope (Zeiss, Hallbergmoos, Germany) at 50 x, 100 x and 400 x magnification. The peribronchiolar area was scored on a scale from 0 to 5: 0, no inflammation; 1, a few inflammatory cells; 2, a layer of inflammatory cells around the structure evaluated; 3, ring of inflammatory cells containing 2 to 4 layers; 4, focal inflammatory cells clustered around the structure; 5, intense inflammatory infiltrate [20]. PAS-positive goblet cells were quantified per 100 μm2 of the PAS stained lungs.
Statistical Analysis
All statistical analysis were performed using Graph Pad prism 5.0 software (GraphPad Software, San Diego, CA). Numerical data were analyzed by the normality test of Kolmogorov-Smirnov. Subsequently, the unpaired t test was used for parametric data and the Mann Whitney test for nonparametric data. The significance level accepted for the tests was P ≤ 0.05. Data are expressed as mean and standard error (mean ± SE).
Results
BCG Treatment Modulates OVA-Induced Airway Inflammation in a BALB/c Mouse Allergy Model
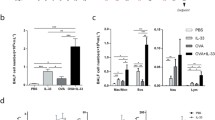
In order to address whether intranasal BCG treatment was able to reduce the airway inflammation, the number of total cells, eosinophils, neutrophils, lymphocytes and macrophages was counted in BAL. EPO activity and CCL11 levels were analyzed in the lungs as an indirect measurement of eosinophil infiltration in this organ. The BCG treatment significantly reduced the infiltration of total cells in BAL (Fig. 2a) as well as the eosinophilic inflammation (Fig. 2b), compared with the OVA group. However, the count of neutrophils did not change between the OVA and the OVA/BCG group (2c). The numbers of total cells and other cell types, except the number of macrophages, were comparable among the PBS and BCG groups (Fig. 2a, b, c, d and e). As regards EPO and CCL11, the BCG-treated allergic mice showed a decrease in EPO activity and CCL11 levels compared with the OVA group (Fig. 2f and g).
OVA-induced airway inflammation is alleviated by BCG treatment. a Total cells, b eosinophils, c neutrophils, d lymphocytes and e macrophages in bronchoalveolar lavage were counted according to stained morphological characteristics. f Eosinophil peroxidase (EPO) and g CCL11 measurement in lungs were performed as described in ‘Materials and methods’. Bars represent the mean ± SE. OD indicates optical density
Cellular Infiltration of Peribronchial Area and Mucus Production Are Inhibited with BCG Treatment
Lung sections were inspected for an overall semiquantitative score of inflammation and mucus production. Ovalbumin sensitization and subsequent airway challenge resulted in intense peribronchial and perivascular inflammatory infiltrates and a mean score of 4 was assigned to the OVA group. When compared with the OVA group, the BCG-treated allergic mice showed reduced inflammatory cell infiltrate (Fig. 3a and c) and mucus production (Fig. 3b), which was confirmed by morphometry analysis with mucus cell counts (Fig. 3d). BCG treatment had no effect on the lung tissue of non-allergic mice, and a mean score less than 1 was assigned to this grooup.
Samples of lung parenchyma of BALB/c mice treated with phosphate-buffered saline (PBS), M. bovis BCG (BCG), ovalbumin (OVA) or ovalbumin plus BCG (OVA/BCG). Lung tissue analysis revealed that BCG treatment reduced OVA-induced airway inflammation (haematoxylin-eosin—HE) a and reduced mucus hypersecretion (Periodic Acid Schiff—PAS) b. Peribronchiolar inflammation and PAS scores are shown in c and d. Five mice per group were analyzed and one image is representative per group. Magnification of images (x40) and scale bar of 20 μm. Bars represent the mean ± SE
Improvement of Airway Inflammation by BCG Treatment Correlates with Immunoglobulin Isotype Shift from OVA-Specific IgE and IgG1
The serum levels of OVA-specific IgE, IgG1 and IgG2a were measured by ELISA. Significantly higher anti-OVA IgE and IgG1 levels were observed in the OVA group compared with the PBS group or the BCG group, indicating allergic immunization of these animals. M. bovis BCG treatment of allergic mice significantly downregulated anti-OVA IgE and IgG1 serum levels (Fig. 4a and b). Additionally, the OVA-specific IgG2a levels in the BCG-treated allergic group were higher than those observed in the OVA group (Fig. 4c).
BCG Treatment Drives an Established Th2 Inflammatory Response Towards a Th1 and T Regulatory Cytokine Production
To determine the manner in which BCG treatment could influence cytokine secretion in the inflammatory site, ELISA was conducted for IL-4, IL-5, IL-13, IFN-γ, TGF-β and IL-10. The BCG treatment of allergic mice resulted in a significant reduction of IL-4 and IL-13 levels (Fig. 5a and c). Also, compared with the OVA group, the BCG-treated allergic group exhibited a significant increase in the levels of IFN-γ, TGF-β and IL-10 (Fig. 5d, e and f). Consistent with these findings, the BCG group showed increased IFN-γ and TGF-β levels, although the other cytokine levels did not alter. In addition, BCG-treated allergic mice had a slightly reduced IL-5 level compared with the OVA group (Fig. 5b).
BCG Treatment Induces Increases in Foxp3 and CTLA-4 Expression by CD4+ T Cells
In order to determine the role of regulatory T cells in a BCG-induced inhibitory effect, the expression of Foxp3 and CTLA-4 by CD4+ T cells was evaluated in the lungs of allergic mice. The BCG-treated mice had a significantly larger number of cells expressing CD4+Foxp3+, compared with the OVA group (Fig. 6a). Additionally, an increase in the number of CD4+CD152+ T cells was also observed in BCG-treated allergic mice (Fig. 6b). Consistent with these results, the BCG group showed an increased expression of Foxp3 and CTLA-4 (CD152) by CD4+ T cells, when compared with the PBS group (Fig. 6a and b).
The beneficial effect of BCG treatment on OVA-induced airway inflammation involves an increased expression of Foxp3, CTLA-4 and IL-10 by lymphocytes. The levels of markers were analyzed by flow cytometry and dot plots depict cells of the lymphocyte region in the forward/side scatter plot. Percentage and frequency of CD4+ cells expressing Foxp3 a, CD152 b, and IL-10 c. d Representative flow cytometry plots of the percentage and frequency of CD8+ cells expressing IL-10. Data are representative of at least two independent experiments and bars represent the mean ± SE
BCG Treatment Induces IL-10-Producing CD8+ T Cells in the Lungs
To further investigate whether the reduced allergen-induced airway inflammation observed in the BCG-treated allergic mice was associated with IL-10-producing regulatory T cells, the intracellular production of this cytokine by lung cells was analyzed. Intriguingly, although the BCG administration to allergic mice did not alter the number of CD4+IL-10+ T cells, a significantly greater number of CD8+IL-10+ T cells were observed (Fig. 6c and d). Interestingly, BCG treatment of non-allergic mice was able to induce significantly IL-10 expression in both CD4+ and CD8+ T cells.
Discussion
The recent increase in the prevalence of allergic disorders, particularly in industrialized countries, provides support for the additional role of environmental factors in the pathogenesis of immune hypersensitivity [21]. Indeed, epidemiological evidence suggests that there is an inverse correlation between the immune response to viral and bacterial infections and the development and prevalence of allergic diseases [6]. In this context, many experimental studies showed that Mycobacterium bovis—Bacille Calmette-Guérin (BCG)—and other mycobacteria are effective in preventing allergic and asthmatic responses, probably through Th1 or regulatory T-cell immune responses [18, 22–24]. However, the therapeutic efficacy of BCG treatment on allergic airway inflammation after allergen challenge remains poorly understood and controversial. In the current study, the effect of giving BCG to mice with previous exposure to allergen was evaluated. The results showed that the mycobacteria treatment was able to inhibit the OVA-induced airway inflammation by reducing the mainly pathogenic allergic features, such as airway eosinophilic inflammation, IgE/IgG1 production by B cells, type 2 cytokine productions and mucus hypersecretion. The reduction of airway pathogenesis to allergen was associated with a switch from Th2-type immune response towards to both Th1 and regulatory T immune responses. Interestingly, the intranasal BCG administration recruited CD4+ T cells expressing Foxp3 and CTLA-4 to the lungs accompanied by an increase in IL-10-producing CD8+ T cells. The protective properties of BCG vaccination were thought to lie mainly in its ability to induce effector CD4 and CD8 T cell responses which secrete cytokines including IFN-γ [25, 26]. In the same way, BCG-driven IFN-γ production has been proposed to shift allergen-specific responses from the Th2 to the Th1 phenotype, subsequently inhibiting the airway inflammatory immune response [27]. In keeping with preview studies [28–32], the present results demonstrated that the improvement of airway inflammation was correlated with an increased IFN-γ production and this was associated with suppression of IL-4, as well with a relevant IgE and IgG1 shift towards to IgG2a protective isotype production. Altogether, these results suggest that, in this allergy model, IFN-γ had an important role as a master regulator of Th2 immune response since it has been well established that this cytokine exerts direct inhibitory effects on Th2 [33] as well as inducing IgG2a instead of IgE production by B cells [34]. On the other hand some studies have demonstrated that treatment with mycobacteria conferred protection against allergen lung inflammation by a mechanism independent of IFN-γ [22, 35]. Probably, this observed apparent discrepancy is due to differences in study design, particularly with regard to the temporal relationship between mycobacteria treatments and allergen exposure.
Besides its Th1 inducer effect, it has been extensively shown that mycobacteria treatment is still able to increase IL-10 and TGF-β production[35–37] and it would be another mechanism by which BCG suppresses the inflammatory processes in mouse model of allergy. In this context, it has been demonstrated that heat-killed M. vaccae treatment improves the pulmonary inflammation without inducing Th1 response [18, 24]. Instead, the protective effect was associated with an induction of CD4+CD45RBlow Treg cells that secrete IL-10 and TGF-β and upon transfer could protect recipient allergic mice from airway inflammation [11].
In line with these findings, the increased levels of IL-10 and TGF-β found in the present study supported the idea that Treg cells have been involved in the BCG-induced inhibitory effect. Therefore, aimed at confirming whether the BCG-modulator effect was associated with inducing of Treg cells, the expression of Foxp3 and CTLA-4 by CD4+ T cells was investigated. Flow cytometry analysis demonstrated that both Foxp3 and CTLA-4 expression were up-regulated in allergic BCG-treated mice, suggesting that cell-cell contact inhibition may be involved in the regulatory mechanism, in addition to the action of IL-10 and TGF-β. These results are similar to those of an earlier study, although the authors of that study evaluated the preventive effect of a neonatal BCG vaccination in inhibiting an allergic eosinophilic inflammation induced in C57BL/6 mice [18], while the present study assessed the ability of BCG to ameliorate an established allergic response in high Th2 responder BALB/c mice. In this respect, the findings are encouraging because modulation of established allergic T-cell responses is a challenge and more difficult than modulating primary responses [38].
Addicionally, an important finding of the current study is that IL-10 production by CD4+ T cells did not change in allergic mice after the BCG treatment, suggesting that other immune cell subsets may be involved in this BCG-induced regulation.
Given the key role of dendritic cells (DCs) in driving and maintaining immune responses to inhaled allergen, it may be possible that mycobacteria interfere with DC-driven Th2-cell sensitization. Indeed, it has recently been demonstrated that CD8α+ and CD8α- DCs subsets from BCG-infected mice produced IL-12 and IL-10 respectively, and the BCG-modulating effect was mediated by both immune deviation and regulation [39]. These data suggest that DCs should be an important coordinator of different BCG-induced allergy inhibition, raising the possibility that DCs might also be a prominent source of the IL-10 detected in this present work. Therefore, further study aimed at evaluating the effect of intra-nasal BCG treatment on DCs activation would be very helpful for understanding the mechanisms by which mycobacteria inhibits allergic inflammation. Finally, since recent reports have shown that CD8+ T lymphocytes may play an important regulatory activity [40], the involvement of these cells was investigated. Surprisingly, the analysis revealed that CD8+ T cells from allergic mice had their IL-10 production increased after BCG administration, suggesting that these cells could be important in dampening down the allergen-induced lung inflammation. In the last few years, some studies have been carried out to shed light on the role of CD8+ T cells in the induction and regulation of inflammatory responses. Recently, it was demonstrated that CD8+ T cell-derived IL-10 diminished disease severity in mice with coronavirus-induced acute encephalitis [40]. In parallel, other studies have shown that the transfer of activated CD8+ T cells from sensitized mice reduces the production of allergen-specific IgE and the development of airway hyperactivity [41, 42]. These regulatory CD8+ T cells could exert their inhibitory effects directly by killing immune cells or indirectly by secreting IL-10 and TGF-β [43, 44]. According to these findings, preview research provided evidence that systemic immunization with allergen induces regulatory CD8+ T cells which were able to inhibit the development of allergic diarrhea by a mechanism that involves IL-10 expression [45].
In spite of these existing correlations, the contribution of CD8+ T cells in the development of allergic airway inflammation remains inconclusive. It was demonstrated that depletion of CD8+ T cells in an early stage of sensitization prevented the development of eosinophilic airway infiltration and allergen-induced airway hyperreactivity [46], but once systemic sensitization is established, CD8+ T cells may play a bystander or pro-inflammatory role in the development of allergic airway disease, since they exhibited a high capacity to produce Th2 cytokines, even though high amounts of IL-10 have been detectable [47]. Therefore, further study in this respect will be helpful for comprehensive elucidation of the role of CD8+ T cell subsets in allergic airway pathogeneses, particularly the relationship between the BCG modulator effect and the induction of regulatory CD8+ T cells.
Conclusions
In conclusion, this study demonstrates that intra-nasal BCG treatment is capable of enhancing an established allergen-driven inflammatory response by markedly hindering the development of the Th2-dependent downstream effector phase of allergy. The results showed evidence of the coexistence of immune deviation and regulatory mechanisms for the modulating effect on Th2-airway inflammation by BCG. The increased production of IFN-γ, IL-10 and TGF-β associated with a higher Foxp3 and CTLA-4 expression seemed to be decisive in this inhibitory effect. Moreover, mycobacteria treatment induces IL-10 production by CD8+ T cells, suggesting participation of other important regulating mechanisms in addition to CD4+ T regulatory cells. Although a therapeutic role for BCG is compatible with the current version of the hygiene hypothesis, analysis of this hypothesis is in progress and the exact mechanism of how BCG exerts its effects remains open. Therefore, further studies should be carried out to elucidate whether it will be possible to use mycobacteria selectively to induce allergen-specific T regulatory cells. Furthermore, new insights into the mechanisms of action of BCG treatment should help to understand the abnormalities of the immune response resulting in allergic diseases.
References
PJ B. Immunology of asthma and chronic obstructive pulmonary disease. Nature reviews Immunology. 2008;8(3):183-92. Epub 2008/02/16.
Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clinical and experimental immunology. 2001;125(2):177–83. Epub 2001/09/01.
Barnes PJ. Pathophysiology of allergic inflammation. Immunological reviews. 2011;242(1):31–50. Epub 2011/06/21.
Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–60. Epub 1989/11/18.
Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. American journal of respiratory and critical care medicine. 2011;184(2):198–205. Epub 2011/04/08.
Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. The New England journal of medicine. 2011;364(8):701–9. Epub 2011/02/25.
Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, et al. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. The Journal of allergy and clinical immunology. 1998;102(5):867–74. Epub 1998/11/18.
Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. The Journal of experimental medicine. 1998;187(4):561–9. Epub 1998/03/28.
Teixeira LK, Fonseca BP, Barboza BA, Viola JP. The role of interferon-gamma on immune and allergic responses. Memorias do Instituto Oswaldo Cruz. 2005;100 Suppl 1:137–44. Epub 2005/06/18.
Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. The Journal of clinical investigation. 1999;103(7):1023–9. Epub 1999/04/09.
Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nature medicine. 2002;8(6):625–9. Epub 2002/06/04.
Robinson DS, Regulatory T. cells and asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2009;39(9):1314–23. Epub 2009/06/23.
Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nature reviews Immunology. 2005;5(4):271–83. Epub 2005/03/19.
Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, et al. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178(3):1433–42. Epub 2007/01/24.
Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nature reviews Immunology. 2011;11(12):852–63. Epub 2011/11/26.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4(4):330–6. Epub 2003/03/04.
Alenmyr L, Matheu V, Uller L, Greiff L, Malm-Erjefalt M, Ljunggren HG, et al. Blockade of CTLA-4 promotes airway inflammation in naive mice exposed to aerosolized allergen but fails to prevent inhalation tolerance. Scandinavian journal of immunology. 2005;62(5):437–44. Epub 2005/11/25.
Li Q, Shen HH. Neonatal bacillus Calmette-Guerin vaccination inhibits de novo allergic inflammatory response in mice via alteration of CD4+CD25+ T-regulatory cells. Acta pharmacologica Sinica. 2009;30(1):125–33. Epub 2008/12/09.
Choi IS, Lin XH, Koh YA, Cui Y. Inoculation route-dependent and allergen-specific suppressive effects of bacille Calmette-Guerin vaccination on asthmatic reactions in BALB/c mice. Lung. 2007;185(3):179–86. Epub 2007/04/05.
Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. International immunopharmacology. 2009;9(4):418–24. Epub 2009/02/03.
Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2008;31(1):143–78. Epub 2008/01/02.
Nahori MA, Lagranderie M, Lefort J, Thouron F, Joseph D, Winter N, et al. Effects of Mycobacterium bovis BCG on the development of allergic inflammation and bronchial hyperresponsiveness in hyper-IgE BP2 mice vaccinated as newborns. Vaccine. 2001;19(11–12):1484–95. Epub 2001/02/13.
Ahrens B, Gruber C, Rha RD, Freund T, Quarcoo D, Awagyan A, et al. BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo. International archives of allergy and immunology. 2009;150(3):210–20. Epub 2009/06/06.
Zuany-Amorim C, Manlius C, Trifilieff A, Brunet LR, Rook G, Bowen G, et al. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J Immunol. 2002;169(3):1492–9. Epub 2002/07/23.
Lagranderie M, Nahori MA, Balazuc AM, Kiefer-Biasizzo H, Silva JR L, Milon G, et al. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology. 2003;108(3):352–64. Epub 2003/02/27.
Koch M, Witzenrath M, Reuter C, Herma M, Schutte H, Suttorp N, et al. Role of local pulmonary IFN-gamma expression in murine allergic airway inflammation. American journal of respiratory cell and molecular biology. 2006;35(2):211–9. Epub 2006/03/18.
Smart JM, Horak E, Kemp AS, Robertson CF, Tang ML. Polyclonal and allergen-induced cytokine responses in adults with asthma: resolution of asthma is associated with normalization of IFN-gamma responses. The Journal of allergy and clinical immunology. 2002;110(3):450–6. Epub 2002/09/05.
Yang X, Fan Y, Wang S, Han X, Yang J, Bilenki L, et al. Mycobacterial infection inhibits established allergic inflammatory responses via alteration of cytokine production and vascular cell adhesion molecule-1 expression. Immunology. 2002;105(3):336–43. Epub 2002/03/29.
Yokoi T, Amakawa R, Tanijiri T, Sugimoto H, Torii Y, Amuro H, et al. Mycobacterium bovis Bacillus Calmette-Guerin suppresses inflammatory Th2 responses by inducing functional alteration of TSLP-activated dendritic cells. International immunology. 2008;20(10):1321–9. Epub 2008/08/16.
Christ AP, Rodriguez D, Bortolatto J, Borducchi E, Keller A, Mucida D, et al. Enhancement of Th1 lung immunity induced by recombinant Mycobacterium bovis Bacillus Calmette-Guerin attenuates airway allergic disease. American journal of respiratory cell and molecular biology. 2010;43(2):243–52. Epub 2009/10/07.
Ou-Yang HF, Hu XB, Ti XY, Shi JR, Li SJ, Qi HW, et al. Suppression of allergic airway inflammation in a mouse model by Der p2 recombined BCG. Immunology. 2009;128(1 Suppl):e343–52. Epub 2009/02/05.
Deng Y, Chen W, Zang N, Li S, Luo Y, Ni K, et al. The antiasthma effect of neonatal BCG vaccination does not depend on the Th17/Th1 but IL-17/IFN-gamma balance in a BALB/c mouse asthma model. Journal of clinical immunology. 2011;31(3):419–29. Epub 2011/02/23.
Sehra S, Pynaert G, Tournoy K, Haegeman A, Matthys P, Tagawa Y, et al. Airway IgG counteracts specific and bystander allergen-triggered pulmonary inflammation by a mechanism dependent on Fc gamma R and IFN-gamma. J Immunol. 2003;171(4):2080–9. Epub 2003/08/07.
Christy AJ, Dharman K, Dhandapaani G, Palaniyandi K, Gupta UD, Gupta P, et al. Epitope based recombinant BCG vaccine elicits specific Th1 polarized immune responses in BALB/c mice. Vaccine. 2012;30(7):1364–70. Epub 2011/12/28.
Adams VC, Hunt JR, Martinelli R, Palmer R, Rook GA, Brunet LR. Mycobacterium vaccae induces a population of pulmonary CD11c+ cells with regulatory potential in allergic mice. European journal of immunology. 2004;34(3):631–8. Epub 2004/03/03.
Bilenki L, Gao X, Wang S, Yang J, Fan Y, Han X, et al. Dendritic cells from mycobacteria-infected mice inhibits established allergic airway inflammatory responses to ragweed via IL-10- and IL-12-secreting mechanisms. J Immunol. 2010;184(12):7288–96. Epub 2010/05/21.
Madura Larsen J, Benn CS, Fillie Y, van der Kleij D, Aaby P, Yazdanbakhsh M. BCG stimulated dendritic cells induce an interleukin-10 producing T-cell population with no T helper 1 or T helper 2 bias in vitro. Immunology. 2007;121(2):276–82. Epub 2007/03/21.
Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nature reviews Immunology. 2008;8(3):218–30. Epub 2008/02/16.
Gao X, Bai H, Cheng J, Fan Y, Wang S, Jiao L, et al. CD8alpha+ and CD8alpha- DC subsets from BCG-infected mice inhibit allergic Th2-cell responses by enhancing Th1-cell and Treg-cell activity respectively. Eur J Immunol. 2012;42(1):165–75. Epub 2011/10/20.
Trandem K, Zhao J, Fleming E, Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J Immunol. 2011;186(6):3642–52. Epub 2011/02/15.
Renz H, Lack G, Saloga J, Schwinzer R, Bradley K, Loader J, et al. Inhibition of IgE production and normalization of airways responsiveness by sensitized CD8 T cells in a mouse model of allergen-induced sensitization. J Immunol. 1994;152(1):351–60. Epub 1994/01/01.
Thomas MJ, MacAry PA, Noble A, Askenase PW, Kemeny DM. T cytotoxic 1 and T cytotoxic 2 CD8 T cells both inhibit IgE responses. Int Arch Allergy Imm. 2001;124(1-3):187–9. Epub 2001/04/18.
Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29(7):337–42. Epub 2008/06/03.
Niederkorn JY. Emerging concepts in CD8(+) T regulatory cells. Curr Opin Immunol. 2008;20(3):327–31. Epub 2008/04/15.
Yamada A, Ohshima Y, Yasutomi M, Ogura K, Tokuriki S, Naiki H, et al. Antigen-primed splenic CD8+ T cells impede the development of oral antigen-induced allergic diarrhea. Allergy Clin Immun. 2009;123(4):889–94. Epub 2009/02/10.
Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, et al. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitization. J Exp Med. 1996;183(4):1719–29. Epub 1996/04/01.
Stock P, Kallinich T, Akbari O, Quarcoo D, Gerhold K, Wahn U, et al. CD8(+) T cells regulate immune responses in a murine model of allergen-induced sensitization and airway inflammation. Eur J Immunol. 2004;34(7):1817–27. Epub 2004/06/24.
Acknowledgments
This study was supported by grants from FAPEMIG (APQ 02164/09), CNPQ (303369/2009-4) and CAPES.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gouveia, A.C.C., Brugiolo, A.S.S., Alves, C.C.S. et al. Th2 Responses in OVA-Sensitized BALB/c Mice Are Down-Modulated By Mycobacterium bovis BCG Treatment. J Clin Immunol 33, 235–245 (2013). https://doi.org/10.1007/s10875-012-9746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9746-4