Abstract
Purpose
Recently, human cytomegalovirus (HCMV) UL128-131 locus gene products have been found to be associated with glycoprotein H (gH) and glycoprotein L (gL) to form a pentameric glycoprotein complex gH/gL/pUL128-130-131, which is present in the virus envelope and elicits production of neutralizing antibodies. Purpose of this study was to verify whether in vitro activities of these antibodies may correlate with protection in vivo.
Methods
By using potently neutralizing human monoclonal antibodies (mAbs) targeting 10 different epitopes of the pentameric complex, a competitive ELISA assay was developed, in which the pentamer bound to the solid-phase was reacted competitively with human sera and murinized human mAbs. In addition, inhibition of virus spreading (plaque formation and leukocyte transfer) by neutralizing human mAbs and sera was investigated.
Results
In the absence of any reactivity of sera from HCMV-seronegative subjects, antibodies to all 10 epitopes were detected in HCMV-seropositive individuals. During primary HCMV infection in pregnancy antibodies to some epitopes showed a trend towards an earlier appearance in mothers not transmitting the virus to the fetus as compared to transmitting mothers. In addition, the activity of neutralizing human mAbs and sera in blocking virus cell-to-cell spreading and virus transfer to leukocytes from infected endothelial cells was shown to develop during the convalescent phase of primary infection.
Conclusions
Dissection of the neutralizing/inhibiting activities of human sera may be helpful in the study of their protective role in vivo. In particular, neutralizing antibodies to the pentamer may be a surrogate marker of protection in vivo.
Similar content being viewed by others
Introduction
Following primary infection, human cytomegalovirus (HCMV) undergoes latency in the presence of a complete immune response including both HCMV-specific antibody and T-cell responses. Subsequent reactivations may be due to defects in the HCMV immune response, with special reference to the cell-mediated arm of the immune response. In this respect, in immunocompromised patients, it has been verified that a HCMV-specific CD8+ T-cell response was able to confer only short-term protection from HCMV disease in the post-transplant period [1, 2], while the simultaneous reconstitution of HCMV-specific CD4+ T-cells was able to confer long-term protection [3, 4], unless episodes of rejection in solid-organ transplant recipients, or graft versus host disease (GvHD) in hematopoietic stem cell transplant recipients, were interfering [5–7].
The role of the humoral arm has been only partially investigated and, thus far, considered of minor importance. However, in recent years administration of HCMV-specific hyperimmune globulin has been claimed to be effective in protecting from HCMV transmission to the fetus and also in treating congenital HCMV disease [8]. Moreover, although the role of Ig administration is controversial [9, 10], a beneficial effect has been reported in the outcome of HCMV infection in transplanted patients [11].
It must be specified that until recently HCMV neutralizing antibodies were measured in the human fibroblast cell test system, and were considered to appear at a low titer late after primary infection, whereas more recently measurement in an epithelial/endothelial cell system has permitted detection of their early appearance at a much higher titer during primary infection [12]. This difference is likely due to the presence in the virus envelope of a pentameric complex (gH/gL/pUL128-130-131) which is required for infection of epithelial/endothelial cells [13, 14]. Major targets of HCMV infection in vivo have been shown to be fibroblasts, epithelial cells, endothelial cells and smooth muscle cells [15]. Recently, we have been able to show that most potently neutralizing monoclonal antibodies (mAbs) are directed to epitopes of the pentameric complex, thus preventing infection of epithelial/endothelial cells, but not fibroblasts [16].
This finding has raised a great deal of renewed interest on the in vivo role of neutralizing Abs. In particular, by taking advantage of the specific reactivity of the isolated human mAbs, 10 distinct neutralization sites were identified within the pentameric complex [16]. In this study, following the development of a competitive ELISA assay, the specific reactivity of human sera with all distinct pentamer sites was investigated. The analysis of this reactivity in different clinical situations should provide significant new data on the role of neutralizing antibodies in protection from HCMV reactivation.
In addition, two important viral biological functions related to viral infection dissemination (cell-to-cell spreading and transfer of virus and viral products from infected endothelial cells to leukocytes) were tested showing an inhibitory effect of both neutralizing human mAbs and human sera against virus dissemination. This inhibitory activity is developing during the convalescent phase of a primary infection [12].
Materials and Methods
Study Population
In this study, HCMV antibodies were tested in the following groups of subjects: i) 13 HCMV-seronegative blood donors; ii) 20 HCMV-seropositive blood donors; iii) 11 pregnant women with primary HCMV infection. The study was approved by the local Bioethics Committee, and informed written consent was obtained. Diagnosis of primary HCMV infection was based on a number of criteria previously reported [17]. In more detail, HCMV-specific IgG and IgM antibodies were determined by ETI-CYTOK-G and ETI-CYTOK-M (DiaSorin, Saluggia. Italy), respectively, while IgG avidity was determined by an in-house developed ELISA test [18]. Sera from pregnant women transmitting and non-transmitting the virus to the fetus were matched as precisely as possible for number of days-post-infection (±2 weeks) and gestational age
Inhibition of mAb Binding (IMAB) Assay
Intronless full-length UL128, UL130, UL131, gH, gL, and gB were cloned into pcDNA3 vector (Invitrogen) by PCR with pfu turbo on cDNA of VR1814-infected MRC-9 cells, using primers introducing the desired restriction sites [16]. Constructs were used to transfect HEK293F cells (Invitrogen) with DNA and polyethylenimine MAX (Polysciences) premixed in Opti-PRO SFM medium (Invitrogen). After 10-day culture, the presence of the proper HCMV glycoprotein complex was verified by ELISA using specific human mAbs. To obtain the secreted soluble forms of the relevant glycoproteins, the gH and gB genes were deprived of the transmembrane portion and the cytoplasmic domain [14, 19]. The gH/gL/pUL128-130-131 complex was obtained by co-transfecting the HEK293 cells with UL128, UL130, UL131, gL, and gH plasmids.
The IMAB assay was designed to study the reactivity of human sera with the previously identified neutralization sites of glycoproteins forming the pentamer complex by using a competitive ELISA binding assay [20]. Following coating of the solid-phase with a human anti-gH (3G16) or anti-pUL130-131 (10P3) mAbs and blocking of non-specific binding sites with 5 % skimmed milk in PBS, the pentamer complex (cell culture supernatant from HEK 293 transfected cells) was captured for 90 min at RT. Subsequently, serum was incubated in serial twofold dilutions (starting from 1:5) for 1 h at RT, prior to adding primary murinized mAb (at a concentration corresponding to 80 % of the maximal reactivity), and then a secondary alkaline phosphatase-labeled goat anti-mouse IgG2a (Southern Biotech, Birmingham, Alabama) for 45 min at RT.
The murinization of human antibodies was performed by cloning the VH and VL/VK genes into expression vectors containing human IgG1, Igκ and Igλ constant regions, in which the human constant region (hinge, CH2 and CH3 domains) was replaced by the homologous mouse IgG2a. Murinized antibodies were produced in transfected HEK 293 cells. The following murinized antibodies (all developed in-house) were used: m15D8, that binds to pUL128 (site 1); m8L13, m5A2, and m10P3, that bind to three non-overlapping epitopes of dimer pUL130-131 (sites 2, 3 and 4); m8J16 and m7I13, that bind to the trimer pUL128-130-131 (sites 5 and 6); m8I21 that recognizes an epitope available on gH/gL/pUL128-130 (site 7); m3G16, m13H11 and mH1P73 that recognize non-overlapping epitopes on gH (site 8, 9 and 10). The principle of this assay is that human sera containing antibodies to a defined neutralization site prevented binding of the relevant murinized site-specific mAb. The % inhibition was calculated as follows: (OD w/o serum-OD w serum) / OD w/o serum – OD background) x 100. A dose–response curve plotting log10 serum dilution vs % inhibition was constructed, from which the serum dilution expressing the IMAB50 titer was interpolated.
Determination of IgG Antibodies to the Pentamer, gH/gL and gB by ELISA
Half-area 96-well polystyrene plates (Corning) were coated overnight with an in-house developed murine anti-gH mAb (mH1P73), or an anti-gB mAb (HCMV 37, Abcam, Cambridge, UK) at a concentration of 0.025–0.050 μg/well and blocked with 5 % skimmed milk in PBS. Following capture of the relevant glycoprotein complex from cell culture supernatants, serial serum twofold dilutions (starting from 1:50) and then horseradish peroxidase-labeled goat IgG fraction to human IgG were added, followed by substrate addition. Net OD was then determined by subtracting OD of serum incubated in the absence of antigen from OD of serum incubated in the presence of antigen.
Neutralization, PFI and LTI Assays
Neutralization assays were performed in 96-well microtiter plates by inoculating virus-serum mixtures (previously incubated for 60 min at 37 °C) in duplicate onto monolayers of epithelial (ARPE-19), endothelial (HUVEC), or fibroblast (HELF) cells. Virus inoculum was 100 PFU in each cell system, using a HUVEC-adapted VR1814 HCMV preparation (titer 1x106,5/ml in HUVEC) at a dilution of 1:1,000 for epithelial cells and 1:4,000 for fibroblasts. Following centrifugation at 700xg for 30 min and 48 h incubation at 37 °C, cells were fixed and stained using a pool of p72 (immediate-early-1 viral protein)-specific murine mAbs [21]. The highest serum dilution inhibiting virus infectivity by 50 % or more compared to virus control was reported as the neutralizing antibody titer.
The plaque formation inhibition (PFI) activity (or cell-to-cell spreading inhibition) was determined in 96-well microtiter plates using the same virus preparation used for the neutralization assay, as follows. After virus adsorption (MOI 0.01) by centrifugation for 30 min at 700xg, ARPE-19 epithelial cell cultures were maintained with medium containing serial serum dilutions for 96 h. Then, cells were fixed and stained with a p72 mAb pool, as above. The 50 % PFI (PFI50) was determined by dividing the difference between the number of IE plaques counted in the absence and in the presence of serum by the number of plaques in the absence of serum (x100). Whenever possible, experiments were performed in triplicate.
Leukocyte transfer inhibition (LTI) experiments were performed in 24-well microtiter plates by incubating human sera at 37 °C with monolayers of 96 h-HCMV-(VR1814)-infected (MOI 1.0) HUVEC 2 h prior to and overnight during co-cultivation with leukocytes [22, 23]. The same virus stock prepared in HUVEC was used. Control experiments were done in the absence of serum. Following co-culture, leukocytes were purified through a Transwell device. Leukocytes were then fixed, permeabilized and stained with a pool of pp65-specific murine mAbs, as reported [24]. The 50 % LTI (LTI50) was calculated by dividing the difference between the number of pp65-positive leukocytes in co-culture experiments in the absence or presence of serum by the number in the absence of serum (x100).
Viral Load Determination
HCMV load in blood of pregnant women was determined by real-time PCR as reported [25].
Results
IMAB Assay by a Competitive ELISA
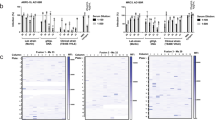
The competitive ELISA assay allowed testing of human sera for the presence/absence of antibodies reactive/not reactive with the 10 epitopes previously identified [16]. The schematic steps of the IMAB assay are reported in Fig. 1a along with the method for the % inhibition titer calculation (Fig. 1b). By using this method, 13 HCMV-seronegative and 20 HCMV-seropositive blood donors were tested against the 10 neutralization sites [16].
Inhibition of monoclonal antibody binding (IMAB) assay. a Schematic of the principle of the assay: antibodies present in human sera compete with murinized human mAbs of known specificity in binding to a previously identified antigenic site (epitope) of the pentamer complex. Percent inhibition was determined as reported at the bottom of the figure. b Determination of the IMAB50 titer against neutralization site 3 (anti-pUL130-131) of sequential human sera drawn from a subject with primary HCMV infection at different intervals post-infection
Results are reported in Fig. 2a for HCMV-seronegative subjects, and in Fig. 2b for the HCMV-seropositive subjects. No reactivity was detected for any of the 10 epitopes in the HCMV-seronegative subjects. Conversely, in the 20 HCMV-seropositive individuals an IMAB50 titer of different level was detected for each of the 10 epitopes tested. From this data, it was inferred that after primary infection an IgG antibody response to nearly all tested neutralization epitopes of the pentameric complex was detected at a titer ranging from <1:5 to 1:640 in all subjects.
The IMAB50 titers to the 10 epitopes of the pentamer previously identified are reported for a group of 13 HCMV-seronegative (a) and a group of 20 HCMV-seropositive blood donors (b). While no reactivity was detected for any of the non-immune individuals, all immune subjects showed variable reactivity with all pentameric epitopes tested
Sequential Appearance of Pentamer Neutralization Site-Specific Antibody Response in Subjects with Primary HCMV Infection
When a series of 11 pregnant women with primary HCMV infection was tested, it was verified that, except for antibodies anti-pUL128, which were generally the first to appear, the antibody response to different epitopes varied in level and time of appearance, according to different individuals. In Fig. 3a and b two cases of primary HCMV infection were tested for their epitope-specific reactivity during the first months after onset of infection. In pt# 1, in the absence of viral DNA in blood, the antibody response to several epitopes began early (15–20 days after onset) reaching a steady state in the following months. As a possible consequence of the early appearance of the neutralizing antibody response, viral DNA was already undetectable in blood at the first time-point tested. In pt. #7, in the presence of viral DNA in blood, the antibody response started later (during the 2nd month) and HCMV clearance from blood was delayed.
The appearance of epitope-specific antibodies (IMAB50 titer) is reported in sequential sera from two subjects with primary HCMV infection. Earlier antibody appearance is seen in subject #1 in the absence of viral DNA in blood, while a later appearance is observed in subject #7 in the presence of viral DNA in blood
In Fig. 4, 11 cases of pregnant women with primary HCMV infection (6 non-transmitting and 5 transmitting the virus to the fetus) were examined during the 2nd month after the onset of infection in the first half of pregnancy. The median day of blood collection was 46 (37–60) days for non-transmitting women and 45 (36–60) days after onset of infection for transmitting women. There was an apparent difference in the kinetics of epitope-specific antibody response observed in the two groups of women, suggesting a trend towards an individual pattern of neutralizing antibody response according to the clinical situation.
PFI and LTI by Human Sera
Two major pathogenetic mechanisms of HCMV dissemination are cell-to-cell spreading within an infected organ (simulating the in vitro plaque formation) [12] and HCMV transfer from infected endothelial cells to circulating leukocytes [22]. In Fig. 5a, the PFI50 activity (inhibition of cell-to-cell spreading), is reported for sequential sera from a subject with primary HCMV infection. In addition, in Fig. 5b the LTI50 is reported for the same subject. PFI50 activity started 6 days after onset of infection (PFI50 titer 1:20), while LTI50 started later, both reaching a 50 % inhibition titer of 1:80–1:160 after 30 days, and a titer of 1:160 or more in the following months. Testing of neutralizing human mAbs showed inhibition of both activities up to a low concentration (data not reported).
Comparison of Different Types of Antibodies Elicited During Primary HCMV Infection
Table I reports the reactivity of different types of HCMV-specific antibodies in sequential sera from two subjects with primary HCMV infection. For comparison, conventional diagnostic parameters (ELISA IgG, IgM, and IgG avidity index) are reported along with neutralizing antibody titers as determined on epithelial (ARPE-19) and fibroblast (HELF) cells. In addition, ELISA IgG titers to viral glycoproteins (pentamer, gH/gL, and gB) along with the number of epitopes reactive in the IMAB assay (whole pentamer, and pUL128-130-131 alone), and the PFI50 and LTI50 antibody titers are reported. IgG antibodies specific for gB appear very early (they are already detected 5 days after onset of infection, patient #2), before IgG specific for the other glycoproteins, and reach higher titers. The titers of IgG antibodies specific for the three glycoprotein complexes increase within the first 3–6 months after infection onset, then reaching a steady level. Similarly, the breadth of the antibody response to the 10 neutralization sites of the gH/gL/pUL128-130-131 complex increases until covering all the defined sites within 3–6 months. The inhibitory effect of sera on the epithelial cell-to-cell spreading is also detected very early after infection, while the ability to block viral transfer to leukocytes (at least within the difference in sensitivities of the two assays) appears to be delayed.
Discussion
This manuscript reports some new methodological approaches to the study of the serum antibody response to HCMV during primary infection, as follows: i) determination of the ELISA IgG antibody response to the pentamer, gH/gL and gB; ii) determination of pentamer neutralization site-specific reactivity of human sera; iii) determination of PFI50 and LTI50 antibody titer.
As for the first point, we recently documented that neutralization of HCMV infection of epithelial/endothelial cells occurs much earlier and at a higher titer than neutralization of infection of human fibroblasts (12). In addition, we isolated a series of neutralizing human mAbs mostly directed to UL128-130-131 locus gene products, which were provided with a neutralizing potency about 3 log10 greater than that of human mAbs directed to gH or gB (16). In addition, neutralizing mAbs appeared to represent the great majority of mAbs directed to the pentamer (Kabanova A., unpublished results). Thus, determination of ELISA IgG antibodies to the pentamer may represent a surrogate marker of neutralizing activity directed to the pentamer.
Secondly, the quantification of the single neutralization epitope reactivity (within the glycoprotein complex gH/gL/pUL128-130-131) of human sera will open a new field of investigation, which appears very promising in view of extending our knowledge on the role of HCMV neutralizing activity. Finally, the determination of antibodies inhibiting virus spreading may help translate in vitro findings into the clinical setting. In a previous study, it was shown that murine mAbs to pUL128 and pUL130 and rabbit antiserum to pUL131 (in combination with each other only, or with gH) showed PFI activity in HUVEC but not in fibroblasts, and LTI activity in both HUVEC and fibroblasts [12]. These findings documented that pUL128-130-131 locus gene products were involved in cell-to-cell spreading in endothelial (and epithelial) cells, but not in fibroblasts. On the other hand, pUL128-130-131 proteins were involved in HCMV transfer to leukocytes either from endothelial/epithelial cells or from fibroblasts. These findings have now been extended to human mAbs and human sera, thus confirming their potential role in limiting virus dissemination.
Our preliminary observation that a different kinetics of epitope-specific IgG antibody appearance seems to be associated with virus transmission to the fetus in transmitter and non-transmitter mothers may help in the understanding of the pathogenesis of congenital HCMV infection/disease. In addition, the severity of CMV infection/disease in the immunocompromised patient may benefit from a better knowledge of the role of neutralizing antibodies both from the pathogenetic and the therapeutic intervention standpoint. Furthermore, the HCMV-seronegative pregnant women, in the absence of an efficacious vaccine, may have the chance of preventing primary infection during pregnancy following administration of hyperimmune globulin preparations rich in potent neutralizing activity. Clinical results in such direction have already been reported [8] and might significantly improve following administration of potently neutralizing human Igs or mAbs.
Finally, the study of the interaction of humoral and cell mediated immunity might represent the best approach to the deeper understanding of the pathogenesis of HCMV infection through the comprehensive study of both the humoral and T-cellular immune response.
References
Sester M, Sester U, Gartner BC, Girndt M, Meyerhans A, Kohler H. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J Am Soc Nephrol. 2002;13:2577–84.
Shlobin OA, West EE, Lechtzin N, Miller SM, Borja M, Orens JB, et al. Persistent cytomegalovirus-specific memory in the lung allograft and responses and blood following primary infection in lung transplant recipients. J Immunol. 2006;176:2625–34.
Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214–22.
Benmarzouk-Hidalgo OJ, Cisneros JM, Cordero E, Martin-Pena A, Sanchez B, Martin-Gandul C, et al. Therapeutic effect of the acquisition of cytomegalovirus-specific immune response during preemptive treatment. Transplantation. 2011;91:927–33.
Gerna G, Lilleri D, Chiesa A, Zelini P, Furione M, Comolli G, Pellegrini C, et al. Virologic and immunologic monitoring of cytomegalovirus to guide preemptive therapy in solid-organ transplantation. Am J Transplant. 2011;11:2463–71.
Gerna G, Lilleri D, Furione M, Baldanti F. Management of human cytomegalovirus infection in transplantation: validation of virologic cut-offs for preemptive therapy and immunological cut-offs for protection. New Microbiol. 2011;34:229–54.
Lilleri D, Fornara C, Chiesa A, Caldera D, Alessandrino EP, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica. 2008;93:248–56.
Nigro G, Adler SP, La Torre R, Best AM, and Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62.
Ljungman P, Cordonnier C, Einsele H, Bender-Götze C, Bosi A, Dekker A, De la Camara R, et al. Use of intravenous immune globulin in addition to antiviral therapy in the treatment of CMV gastrointestinal disease in allogeneic bone marrow transplant patients: a report from the European Group for Blood and Marrow Transplantation (EBMT). Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 1998;21:473–6.
Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2008;4:CD006501.
Bonaros N, Mayer B, Schachner T, Laufer G, Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant. 2008;22:89–97.
Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, Gallina A, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89:853–65.
Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–33. Erratum in: J Virol. 2009;83:6323.
Ryckman BJ, Rainish BL, Chase MC, Borton JA, Nelson JA, Jarvis MA, Johnson DC. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008;82:60–70.
Sinzger C, Grefte A, Plachter B, Gouw ASH, The TH, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–50.
Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2010;84:1005–13.
Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15:680–715.
Revello MG, Genini E, Gorini G, Klersy C, Piralla A, Gerna G. Comparative evaluation of eight commercial human citomegalovirus IgG avidity assays. J Clin Virol. 2010;48:255–9.
Carlson C, Britt WJ, Compton T. Expression, purification, and characterization of a soluble form of human cytomegalovirus glycoprotein B. Virology. 1997;239:198–205.
Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5:e8805.
Gerna G, Baldanti F, Percivalle E, Zavattoni M, Campanini G, Revello MG. Early identification of human cytomegalovirus strains by the shell vial assay is prevented by a novel amino acid substitution in UL123 IE1 gene product. J Clin Microbiol. 2003;41:4494–5.
Gerna G, Percivalle E, Baldanti F, Sozzani S, Lanzarini P, Genini E, Lilleri D, Revello MG. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J Virol. 2000;74:5629–38.
Revello MG, Baldanti F, Percivalle E, Sarasini A, De-Giuli L, Genini E, Lilleri D, et al. In vitro selection of human cytomegalovirus variants unable to transfer virus and virus products from infected cells to polymorphonuclear leukocytes and to grow in endothelial cells. J Gen Virol. 2001;82:1429–38.
Gerna G, Revello MG, Percivalle E, Morini F. Comparison of different immunostaining techniques and monoclonal antibodies to the lower matrix phosphoprotein (pp65) for optimal quantitation of human cytomegalovirus antigenemia. J Clin Microbiol. 1992;30:1232–7.
Gerna G, Vitulo P, Rovida F, Lilleri D, Pellegrini C, Oggionni T, Campanini G, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78(3):408–16. Erratum in: J Med Virol. 2008;80:1869.
Acknowledgments
The authors thank the technical staffs of the two collaborating laboratories. In addition, they like to thank Daniela Sartori for careful manuscript editing and Laurene Kelly for revision of the English. This work was partially supported by grants from Fondazione CARIPLO, Milan, Italy (grant 93043/A), and by Fondazione Carlo Denegri, Turin, Italy.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lilleri, D., Kabanova, A., Lanzavecchia, A. et al. Antibodies Against Neutralization Epitopes of Human Cytomegalovirus gH/gL/pUL128-130-131 Complex and Virus Spreading May Correlate with Virus Control In Vivo . J Clin Immunol 32, 1324–1331 (2012). https://doi.org/10.1007/s10875-012-9739-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9739-3