Abstract
Introduction
CD8+ lymphocytes can suppress HIV replication without killing the infected cells. This CD8+ cell noncytotoxic anti-HIV response (CNAR) is associated with a beneficial clinical course.
Materials and Methods
In this longitudinal study of 16 participants in the Options Project at UCSF, we measured the ability of CD8+ lymphocytes to suppress HIV replication in CD4+ cells during primary HIV infection, early antiretroviral therapy, and after treatment.
Results and Discussion
CD8+ lymphocytes from subjects with untreated primary HIV-1 infection strongly suppressed HIV replication. Initiation of antiretroviral therapy during primary HIV-1 infection caused a marked decline in this CNAR. CD8+ cells from these subjects regained anti-HIV activity when early therapy was discontinued. The timing of the appearance of CD8+ cell anti-HIV activity directly correlated with the emergence of detectable virus levels. Maximal CNAR activity coincided with a decay in the kinetics of HIV replication. In addition, peak viral loads during treatment interruption were lower than pre-treatment virus levels (median reduction = 0.8 logs, p = 0.005) and CD4+ T cell counts were maintained for a 24-week period of follow-up.
Conclusion
These results suggest that CNAR plays an important role in suppressing HIV replication in the setting of antiretroviral treatment interruption in HIV-infected individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major objective of the Options Project at the University of California San Francisco (UCSF) has been to evaluate the potential benefits of initiating antiretroviral therapy (ART) very early in the course of HIV infection [1]. During the past 10 years, 544 subjects undergoing primary HIV-1 infection were enrolled in the Options Project. Close observation of these participants, including 314 subjects who elected to initiate ART immediately or within 1 year of enrollment, has allowed for the clinical, virologic, and immunologic characterization of early treatment [1]. Notable features of early ART include the durable suppression of HIV replication, the maintenance of CD4+ cell numbers [2], preservation of B- and T-cell repertoire diversity [3, 4], and the waning of anti-HIV immune responses [5, 6].
The periodic application of ART continues to be evaluated for its potential clinical benefits. It has been speculated that structured treatment interruption (STI) may have an autovaccination-like effect and thereby enhance anti-HIV immune responses [7]. STI has been most carefully studied in the subjects with chronic and advanced HIV infection and several reports have noted that, in this context, some individuals rapidly progress to disease [8–11]. Thus, STI protocols appear to be contraindicated for subjects who initiate ART during the chronic and advanced stages of HIV infection. However, STI in subjects treated during early HIV infection (STIe) may have a more positive outcome [12–15].
Numerous studies have described the anti-HIV functions of CD8+ lymphocytes and their importance in HIV infection [16–21]. Distinct from their classical cytotoxic function, CD8+ cells can suppress HIV replication through noncytotoxic mechanisms [17, 18, 21–24]. The CD8+ cell noncytotoxic anti-HIV response (CNAR) is particularly relevant because of its direct correlation with a healthy clinical state [25–29]. Asymptomatic long-term survivors of HIV infection have strong CNAR activity, whereas this response is lost with progression to disease [26–32]. Additional features of CNAR include (1) its effectiveness on divergent strains and subtypes of HIV [22, 28, 33], (2) its specific effect of blocking LTR-driven transcription [34–36], and (3) its association with the secretion of the CD8+ cell antiviral factor (CAF) [18, 36–38]. Therapeutic approaches that enhance CNAR/CAF might be beneficial to HIV-infected individuals.
The objective of this study was to evaluate the CD8+ cell noncytotoxic anti-HIV response in subjects that discontinued antiretroviral therapy that was initiated during primary HIV-1 infection. The results from this study provide insights into the relationships between pre-existing CD8+ cell antiviral activity and emerging HIV replication.
Materials and Methods
Human Subjects
The 16 subjects in this study (Table I) were enrolled in the Options Project at UCSF upon a diagnosis of primary HIV-1 infection [39, 40]. These subjects initiated standard antiretroviral treatment (ART; e.g., 300 mg zidovudine, 150 mg lamivudine, and 1250 mg nelfinavir twice daily or a similar regimen) within 6 months of their estimated infection dates. After maintaining HIV RNA plasma viral loads (VL) below 50 copies/ml for at least 16 weeks, these subjects elected to discontinue ART and participate in this study of structured treatment interruption approved by the UCSF Committee on Human Subjects.
HIV-1 viral load measurements and clinical blood cell counts
Plasma viral loads (HIV-1 RNA copies /ml) were measured using the branched-chain DNA test (Seimens Diagnostics, Emeryville, CA) [41]. Complete differential blood cell counts for red cell number, hemoglobin, total leukocytes, granulocytes, lymphocytes, monocytes, platelets, and T cell subsets were performed by Quest Laboratories (San Jose, CA).
Cell specimens
Whole-blood samples were collected in evacuated blood tubes (Vacutainer, Becton Dickinson, San Jose, CA) containing acid–citrate–dextrose at regular intervals during the course of the study. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation, cryopreserved in fetal bovine serum (FBS) containing 10% DMSO, and stored in ultra-low-temperature freezers before use.
CD8+ cell antiviral responses
CD8+ cell noncytotoxic anti-HIV activity was assessed as the ability of CD8+ cells to suppress HIV replication in primary CD4+ cells as previously described [21, 29]. All cell specimens from each subject were thawed and processed simultaneously. We have previously determined that cryopreservation does not substantially affect CNAR activity [27]. Briefly, CD4+ and CD8+ cells were serially isolated by positive selection using immunomagnetic beads (Miltenyi, Oslo, NO) from the PBMC of the study subjects and healthy uninfected blood donors. The CD4+ cells were stimulated with PHA (3 μg/ml, Sigma) for 3 days and then acutely infected with the β-chemokine-resistant strain HIV-1SF33 (104 TCID50 per 107 cells per ml) [25, 29, 42, 43]. Unstimulated CD8+ cells were cocultured with autologous HIV-infected CD4+ cells at ratios of 0.25:1, 0.5:1, 1:1 in a total volume of 200 μl of complete medium (RPMI medium 1640, 10% FBS, antibiotics, 100 U/ml rIL-2, and 2 mM l-glutamine). When sufficient numbers of cells were available, CD8+ cells were also cocultured with acutely infected heterologous CD4+ cells. Duplicate wells were established in 96-well flat-bottom plates. HIV replication levels were determined by measuring the reverse transcriptase (RT) activity present in the cell culture supernatants as described previously [44]. CNAR activity (percent suppression) was evaluated as a ratio of the RT activity in wells containing CD4+ cells cultured in the presence and absence of CD8+ cells.
Statistical Analysis
Data were compiled in an Access database (Microsoft, Seattle, WA). Descriptive values and statistical comparisons were computed using S-PLUS 6.1 (Insightful, Seattle, WA). Graphs were prepared using SigmaPlot 8 (Systat, San Jose, CA).
Results
Demographic and clinical characteristics of the subjects electing to discontinue early therapy
All 16 participants in this study (Table I) were men who reported having sex with men as their primary risk of HIV infection. The median age at the time of infection was 37 years (range = 27–59 years). Among these men, ART was initiated at a median of 79 days (range = 30–180 days) following the estimated date of infection. Due to decreased CD4+ T cell counts and/or elevated CD8+ T cell levels, 13 of the 16 subjects exhibited an inverted T cell ratio at the time ART was initiated. At the time therapy was discontinued, each subject had an undetectable plasma virus level and only one subject exhibited an inverted T cell ratio. The median duration of ART prior to STIe was 3.4 years (range = 0.9–4.8 years).
Changes in viral load and T cell counts during STIe
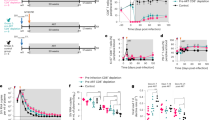
To evaluate the effects of discontinuing early treatment on clinical parameters, longitudinal measurements of HIV viral load, CD4+ T cell, and CD8+ T cell counts were analyzed (Fig. 1). Following cessation of treatment, the median elapsed time at which the HIV viral load exceeded 500 RNA copies/ml was 4 weeks (interquartile range, IQR = 3–5 weeks). Viral loads were generally observed to reach a peak level 4–8 weeks post-treatment (Fig. 1a). In comparison to pre-treatment virus levels, maximum viral loads in the first 8 weeks of STIe were lower in 15 of 16 subjects (Fig. 1b, median reduction = 0.8 logs, p = 0.005, paired t-test). The one exception was a subject (S01) who initiated therapy without ever having a detectable viral load. Seven of the nine subjects who discontinued early ART for at least 24 weeks experienced modest CD4+ cell declines during the first 8 weeks of STIe (Fig. 1c, mean decline = 27%, p = 0.013, paired t-test). However, this loss of CD4+ cells appeared to be transient, as only four of the nine subjects exhibited lower than treatment levels of CD4+ cells at week 24 of STIe. While their CD4+ T cell levels remained relatively stable over the 24-week STIe interval, the study subjects exhibited an expansion of CD8+ T cells (Fig. 1d) during this period. Significantly elevated CD8+ cell counts became apparent at week 16 (paired t-test, p = 0.011) and persisted at week 24 (p = 0.016) of STIe.
Changes in viral load and T cell counts during STIe. a The kinetics of HIV replication upon discontinuation of early ART are shown for the 16 study subjects. Nonlinear regression analyses were performed using a four-parameter sigmoidal function (dotted line) and a three-period mixed model (solid line). b Comparisons of viral loads at the time ART was initiated (Txi), discontinued (ART), and at the peak virus level during the first 8 weeks of STIe (STIe). Changes in c CD4+ T cell and d CD8+ T cell counts are shown for subjects during STIe. In each plot, each subject is denoted by a distinct symbol
Ten of the 16 subjects in this study resumed antiretroviral therapy following STIe. In each case, sub-detection levels of virus (<50 HIV RNA copies/ml) were reached and maintained (data not shown). No occurrences of drug-resistant virus replication were noted during the follow-up period.
Longitudinal measurements of CD8+ cell anti-HIV activity
The ability of bulk unstimulated CD8+ cells to suppress HIV replication in acutely infected primary CD4+ cells was measured among 16 subjects at multiple time points during the course of primary HIV-1 infection, early antiretroviral therapy, and upon cessation of therapy (Fig. 2). Although the study was designed to focus on the first 24 weeks of treatment interruption, we were able to measure CD8+ cell anti-HIV activity for longer periods of time following treatment (years in some cases) among several of the study subjects. The expected cytopathic effects (CPE e.g., cell ballooning, syncytia, and cell death) of the CXCR4-tropic HIV-1SF33 strain used in our assays were visually apparent in cultures lacking CD8+ cell antiviral activity. In cultures showing no CPE, HIV quickly replicated and CPE became evident upon removal of the CD8+ cells. This observation demonstrated that HIV-infected cells persist in the presence of CNAR activity as reported [17, 35]. This CD8+-cell-mediated suppression of HIV replication was also observed in cultures containing heterologous CD4+ cells (Fig. 2) [45]. Together, these observations support the presence of noncytotoxic and β-chemokine independent CD8+ cell antiviral activity in the setting of early treatment interruption.
Longitudinal measurements of CD8+ cell noncytotoxic anti-HIV activity. Shown are representative measurements of CNAR activity (left y-axis) in cocultures containing 1:1 input ratios of CD8+ cells and autologous (green circles) or heterologous (purple squares) acutely infected CD4+ cells. Viral load values (red diamonds) are indicated on the right y-axis. For each of the four subjects shown, ART periods are shaded on the x-axis
Effect of early treatment and its discontinuation on CD8+ cell anti-HIV activity
In evaluating the effect of antiretroviral therapy on the CD8+ cell noncytotoxic anti-HIV response, CNAR activity was measured prior to treatment and during the course of ART (Fig. 3a). The availability of pre-treatment cell specimens allowed for assessments of 13 of the 16 study subjects. During the course of treatment, CD8+ cells from each of these subjects exhibited a substantial decrease in anti-HIV activity, with the exception of one subject (S04) whose CD8+ cells exhibited no pre-treatment anti-HIV activity. Noteworthy is that this subject displayed the highest viral load at the time of treatment initiation (see Table I). None of the 16 subjects studied exhibited appreciable CNAR activity at the time treatment was discontinued (week 0, Fig. 3b). Our data suggest that the CD8+ cell anti-HIV response is diminished within weeks to months upon treatment, although the availability of specimens proximal to treatment initiation limited our ability to precisely determine the rate of loss of this response.
Effects of early antiretroviral therapy and its discontinuation on CD8+ cell anti-HIV activity. a Shown is the ability of CD8+ cells from 13 subjects to suppress HIV replication (1:1 CD8+ cell to acutely infected autologous CD4+ cell culture input ratios) during untreated primary HIV-1 infection (week 0) and following the initiation of early antiretroviral therapy. b Shown are longitudinal changes in CNAR activity during STIe. The dark solid line reveals results of a nonlinear regression analysis using a four-parameter sigmoidal function. For panels a and b, CD8+ cells from individual subjects are distinguished by unique symbols. c Co-plotted are viral loads and CD8+ cell anti-HIV activity levels at weeks 0 (triangles), 4 (squares), and 8–24 (circles)
To determine the effect of discontinuing early antiretroviral therapy, for each of the 16 subjects studied, CNAR activity was measured during ART and treatment interruption. One or more measurements of CNAR activity were performed on CD8+ cells from 15 of the 16 subjects during the first 8 weeks of treatment interruption (Fig. 3b). Each subject exhibited a dramatic increase in CD8+ cell anti-HIV activity during this STIe period. Nonlinear regression analyses indicated that peak levels of CD8+ cell anti-HIV activity were reached approximately 4 weeks post ART. The strength of CNAR activity was statistically assessed by evaluating the percent suppression at graduated cell culture input ratios (e.g., 1:1, 1:2, or 1:4 ratios of CD8+ cells to CD4+ cells) and by summing the percent suppression values across cell culture input ratios (e.g., 1:1 + 1:2 values). These approaches did not reveal any appreciable differences in the strength of CD8+ cell antiviral activity between study subjects during STIe (data not shown).
Association between viral load and CD8+ cell noncytotoxic anti-HIV activity
Having separately observed reduced virus levels (Fig. 1b) and strong CD8+ cell HIV-suppressing activity (Fig. 3b) during STIe, we formally assessed the relationship between viral load and CNAR. These variables were stratified by time and then coplotted (Fig. 3c). A direct correlation between viral load and CD8+ cell anti-HIV activity was evident. Although variations in viral loads were observed between subjects during STIe (Fig. 1a), circulating virus levels increased in all 16 participants during STIe and exhibited a typical kinetics pattern with peak levels occurring approximately 4 weeks post ART. Analyses of CNAR activity revealed that very strong responses had returned by week 4 of STIe in most subjects (Fig. 3b). Thus, the timing of the return of maximal CD8+ cell anti-HIV activity (weeks 4–8) coincided with the timing of the decay in the HIV replication rate (Fig. 3).
Discussion
We hypothesized that the early application of antiretroviral therapy could reduce HIV levels and preserve immune functions, thereby promoting the CD8+ cell noncytotoxic anti-HIV response (CNAR) upon treatment interruption. In this longitudinal study of 16 subjects (Table I), we measured the ability of CD8+ lymphocytes to suppress HIV replication at time points during acute and primary HIV-1 infection, early treatment, and upon discontinuation of ART. CNAR activity, measured in more than 200 clinical blood specimens, was evaluated with respect to HIV viral load and CD4+ T cell count.
In this study, we observed two positive clinical outcomes of STIe (Fig. 1). First, peak plasma HIV RNA levels, occurring at 4–8 weeks of treatment interruption (Fig. 1a), were lower than those observed during untreated primary HIV-1 infection (Fig. 1b). The observed peak viral loads during STIe were likely much lower than the theoretical peak viral loads occurring during the initial weeks of infection and prior to study enrollment [39]. Second, during 24 weeks of STIe, circulating CD4+ T cell counts remained relatively stable (Fig. 1c), whereas CD8+ T cells levels substantially increased (Fig. 1d). These findings agree with observations in a recent report [15] and implicate a role for a beneficial immune response(s) during STIe. Our longitudinal analyses of CNAR activity demonstrated that this response is associated with the observed positive clinical outcomes.
The effects of early antiretroviral therapy and its discontinuation on CNAR activity were remarkably consistent (Fig. 2). Upon initiation of early ART (Fig. 3a), the CD8+ cell anti-HIV activity, commonly found in subjects with untreated acute and primary infection, decreased. In a previous study of mitogen-stimulated CD8+ cells, we observed that CNAR activity, in primary HIV infection subjects receiving early antiretroviral therapy, declined in association with reduced viremia [5]. A similar reduction in CNAR activity has been reported to occur in persons starting ART during chronic HIV-1 infection [46]. In the present study using unstimulated CD8+ cells, the effect of ART appeared to be more substantial and universal. The observed differences in the sizes of the effects between these studies suggest that unstimulated CD8+ cells can provide a more sensitive measurement of CNAR activity.
Another consistently observed pattern was that CNAR activity quickly returned during STIe (Fig. 3b). All 16 subjects studied experienced a rebound in CD8+ cell anti-HIV activity during STIe. Importantly, this antiviral response returned at approximately week 4 of STIe and coincided with a tapering of virus replication levels in the blood (week 4, Fig. 1a). Although many of the subjects resumed antiretroviral therapy before reaching a clear viral load set point during STIe, we observed that the peak viral loads during STIe, occurring 4–8 weeks post-treatment, were lower than the viral loads at the time treatment was initiated (Fig. 1b). The subjects who resumed antiretroviral therapy early in the course of STIe had been randomized to a group having a very low limit (1,000 HIV RNA copies/ml) on the allowable plasma viral load. Thus, the virologic and immunologic responses of those subjects were not necessarily different from the other participants.
Because of the limited availability of specimens at crucial time points, we were unable to directly establish (1) whether or not CNAR activity was elevated during STIe in comparison to pre-treatment levels and (2) the precise relationship between virus replication levels and CNAR activity during the first weeks of STIe. However, the return of maximal CNAR activity was found to be temporally correlated with decayed virus replication kinetics during STIe (Fig. 3c), suggesting that this response plays a beneficial immunologic role in this setting. We speculate that immune-based therapies that can preserve CNAR activity during early ART, such as the use of IL-2 [47] or IL-15 [48] treatment, may result in an additional reduction in viral load during STIe.
In summary, we report an association between strong CD8+ cell noncytotoxic anti-HIV activity and reduced virus replication levels following the discontinuation of early antiretroviral therapy. Additional studies are needed to establish optimal treatment interruption strategies for enhancing CD8+ cell immune responses that can further reduce HIV replication levels. At the same time, the potential complementary contributions of other immune responses need to be considered.
References
Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194(6):725–33. doi:10.1086/506616.
Killian MS, Fujimura SH, Hecht FM, et al. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS 2006;20(9):1247–52. doi:10.1097/01.aids.0000232231.34253.bd.
Elkins MK, Vittinghoff E, Baranzini SE, et al. Longitudinal analysis of B cell repertoire and antibody gene rearrangements during early HIV infection. Genes Immun. 2005;6(1):66–9.
Schito AM, Vittinghoff E, Hecht FM, et al. Longitudinal analysis of T-cell receptor gene use by CD8(+) T cells in early human immunodeficiency virus infection in patients receiving highly active antiretroviral therapy. Blood 2001;97(1):214–20. doi:10.1182/blood.V97.1.214.
Stranford SA, Ong JC, Martinez-Marino B, et al. Reduction in CD8+ cell noncytotoxic anti-HIV activity in individuals receiving highly active antiretroviral therapy during primary infection. Proc Natl Acad Sci USA. 2001;98(2):597–602. doi:10.1073/pnas.021550598.
Killian MS, Norris PJ, Rawal BD, et al. The effects of early antiretroviral therapy and its discontinuation on the HIV-specific antibody response. AIDS Res Hum Retroviruses. 2006;22(7):640–7. doi:10.1089/aid.2006.22.640.
Lisziewicz J, Rosenberg E, Lieberman J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340(21):1683–4. doi:10.1056/NEJM199905273402114.
Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS 1999;13(8):F59–62. doi:10.1097/00002030-199905280-00001.
Garcia F, Plana M, Vidal C, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS 1999;13(11):F79–86. doi:10.1097/00002030-199907300-00002.
Ruiz L, Martinez-Picado J, Romeu J, et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS 2000;14(4):397–403. doi:10.1097/00002030-200003100-00013.
El Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi:10.1056/NEJMoa062360.
Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature 2000;407(6803):523–6. doi:10.1038/35035103.
Hel Z, Venzon D, Poudyal M, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med. 2000;6(10):1140–6. doi:10.1038/80481.
Lori F, Maserati R, Foli A, et al. Structured treatment interruptions to control HIV-1 infection. Lancet 2000;355(9200):287–8. doi:10.1016/S0140-6736(99)03515-1.
Steingrover R, Pogany K, Fernandez GE, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS 2008;22(13):1583–8.
Kostense S, Raaphorst FM, Joling J, et al. T cell expansions in lymph nodes and peripheral blood in HIV-1-infected individuals: effect of antiretroviral therapy. AIDS 2001;15(9):1097–107. doi:10.1097/00002030-200106150-00004.
Walker CM, Moody DJ, Stites DP, et al. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 1986;234(4783):1563–6. doi:10.1126/science.2431484.
Walker CM, Levy JA. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology 1989;66(4):628–30.
Borrow P, Lewicki H, Hahn BH, et al. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68(9):6103–10.
Cocchi F, DeVico AL, Garzino-Demo A, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995;270(5243):1811–5. doi:10.1126/science.270.5243.1811.
Mackewicz CE, Yang LC, Lifson JD, et al. Non-cytolytic CD8 T-cell anti-HIV responses in primary HIV-1 infection. Lancet 1994;344(8938):1671–3. doi:10.1016/S0140-6736(94)90459-6.
Walker CM, Erickson AL, Hsueh FC, et al. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J Virol. 1991;65(11):5921–7.
Yang OO, Kalams SA, Trocha A, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71(4):3120–8.
Mackewicz C, Levy JA. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS Res Hum Retroviruses. 1992;8(6):1039–50.
Barker E, Mackewicz CE, Reyes-Teran G, et al. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood 1998;92(9):3105–14.
Gomez AM, Smaill FM, Rosenthal KL. Inhibition of HIV replication by CD8+ T cells correlates with CD4 counts and clinical stage of disease. Clin Exp Immunol. 1994;97(1):68–75.
Landay AL, Mackewicz CE, Levy JA. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69(1):106–16. doi:10.1006/clin.1993.1157.
Levy JA, Mackewicz CE, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17(5):217–24. doi:10.1016/0167-5699(96)10011-6.
Mackewicz CE, Ortega HW, Levy JA. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87(4):1462–6. doi:10.1172/JCI115153.
Blackbourn DJ, Mackewicz CE, Barker E, et al. Suppression of HIV replication by lymphoid tissue CD8+ cells correlates with the clinical state of HIV-infected individuals. Proc Natl Acad Sci USA. 1996;93(23):13125–30. doi:10.1073/pnas.93.23.13125.
Castelli JC, Deeks SG, Shiboski S, et al. Relationship of CD8(+) T cell noncytotoxic anti-HIV response to CD4(+) T cell number in untreated asymptomatic HIV-infected individuals. Blood 2002;99(11):4225–7. doi:10.1182/blood-2001-11-0078.
Walker CM, Moody DJ, Stites DP, et al. CD8+ T lymphocyte control of HIV replication in cultured CD4+ cells varies among infected individuals. Cell Immunol. 1989;119(2):470–5. doi:10.1016/0008-8749(89)90259-1.
Walker CM, Thomson-Honnebier GA, Hsueh FC, et al. CD8+ T cells from HIV-1-infected individuals inhibit acute infection by human and primate immunodeficiency viruses. Cell Immunol. 1991;137(2):420–8. doi:10.1016/0008-8749(91)90090-X.
Bonneau KR, Ng S, Foster H, et al. Derivation of infectious HIV-1 molecular clones with LTR mutations: sensitivity to the CD8+ cell noncytotoxic anti-HIV response. Virology 2008;373(1):30–8. doi:10.1016/j.virol.2007.11.003.
Mackewicz CE, Blackbourn DJ, Levy JA. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92(6):2308–12. doi:10.1073/pnas.92.6.2308.
Chen CH, Weinhold KJ, Bartlett JA, et al. CD8+ T lymphocyte-mediated inhibition of HIV-1 long terminal repeat transcription: a novel antiviral mechanism. AIDS Res Hum Retroviruses. 1993;9(11):1079–86.
Levy JA. The search for the CD8+ cell anti-HIV factor (CAF). Trends Immunol. 2003;24(12):628–32. doi:10.1016/j.it.2003.10.005.
Brinchmann JE, Gaudernack G, Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990;144(8):2961–6.
Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 2002;16(8):1119–29. doi:10.1097/00002030-200205240-00005.
Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 1998;280(1):42–8. doi:10.1001/jama.280.1.42.
Elbeik T, Charlebois E, Nassos P, et al. Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5. J Clin Microbiol. 2000;38(3):1113–20.
Tateno M, Levy JA. MT-4 plaque formation can distinguish cytopathic subtypes of the human immunodeficiency virus (HIV). Virology 1988;167(1):299–301. doi:10.1016/0042-6822(88)90084-0.
Levy JA, Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985;152(4):734–8.
Hoffman AD, Banapour B, Levy JA. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology 1985;147(2):326–35. doi:10.1016/0042-6822(85)90135-7.
Mackewicz CE, Garovoy MR, Levy JA. HLA compatibility requirements for CD8(+)-T-cell-mediated suppression of human immunodeficiency virus replication. J Virol. 1998;72(12):10165–70.
Wilkinson J, Zaunders JJ, Carr A, et al. CD8+ anti-human immunodeficiency virus suppressor activity (CASA) in response to antiretroviral therapy: loss of CASA is associated with loss of viremia. J Infect Dis. 1999;180(1):68–75. doi:10.1086/314833.
Martinez-Marino B, Shiboski S, Hecht FM, et al. Interleukin-2 therapy restores CD8 cell non-cytotoxic anti-HIV responses in primary infection subjects receiving HAART. AIDS 2004;18(15):1991–9. doi:10.1097/00002030-200410210-00003.
Castelli J, Thomas EK, Gilliet M, et al. Mature dendritic cells can enhance CD8+ cell noncytotoxic anti-HIV responses: the role of IL-15. Blood 2004;103(7):2699–704. doi:10.1182/blood-2003-06-1913.
Acknowledgments
The research presented in this report was supported by National Institutes of Health Grant U010AI-41531. The authors gratefully thank the Options Project staff members and the study participants for their dedicated support of this research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Killian, M.S., Roop, J., Ng, S. et al. CD8+ Cell Anti-HIV Activity Rapidly Increases Upon Discontinuation of Early Antiretroviral Therapy. J Clin Immunol 29, 311–318 (2009). https://doi.org/10.1007/s10875-009-9275-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9275-y