Abstract
Background
Establishment of immunological memory is a hallmark of adaptive immune responses and the biological mechanism for the success of vaccines. However, in humans, much of our knowledge about adaptive immune responses derives from studies of chronic viral infections.
Objective
Here, we summarize the work of our laboratory and others on B and T cell responses and the establishment and maintenance of immune memory after acute viral infections induced by vaccination with two of the most successful vaccines to date, the yellow fever and the smallpox vaccines.
Similar content being viewed by others
Introduction
A central feature of adaptive immunity is the establishment of immunological memory. After the initial encounter with a pathogen or vaccine, B and T cells specific for the pathogen expand and perform effector functions. Following resolution of the infection, the immune system maintains a population of B and T cells that is substantially larger than the initial pool of pathogen-specific cells. In contrast to naive B and T cells, these memory cells respond immediately upon subsequent exposure to the pathogen. The kinetics of the memory recall response is not only faster than a primary response, but also of a greater magnitude and efficacy. Established immune memory can either prevent reinfection all together or at least reduce the severity of disease upon re-exposure to a pathogen. Immunological memory is comprised of a cellular and a humoral component. Soluble antibodies produced by long-lived plasma cells (LLPC) maintain a first line of defense both systemically and at mucosal surfaces. In contrast, memory B cells are able to provide more antibody-producing cells if needed and may also be involved in maintaining the numbers of LLPCs. Whereas antibody-mediated immunity can protect from initial infection or reduce the spread of the pathogen, CD8 T cells destroy already infected cells by a range of effector mechanisms. To gain better understanding of the kinetics, magnitude, and quality of the adaptive immune response and the resulting memory pool, we have studied the human B and T cell immune responses to acute viral infection, using the potent and highly successful live viral vaccines against yellow fever (YF-Vax) and smallpox (Dryvax). This paper summarizes results from these studies.
Human B Cell Responses after Yellow Fever Virus or Smallpox Vaccination
Infection or vaccination in humans induces prolonged antibody production lasting for years and, in some cases, for life, for example, after smallpox or yellow fever vaccination [1–4] (Fig. 1). In many cases, these antibodies are protective and provide an important first line of defense against pathogenic exposure [5]. In the case of the above-mentioned primary smallpox vaccination, we find that neutralizing antibody titers are readily detected in serum 10–14 days postvaccination, with peak levels present by 1 month postvaccination [6]. An antibody response with similar kinetics was observed after yellow fever virus vaccination. Cross-sectional studies of smallpox vaccinees have shown that, after an initial drop over the first few years postvaccination, the antibody titers can be maintained for as much as 75 years after smallpox vaccination [3, 4]. Further, using newly developed protein array technology, we could analyze the breadth of these antibody responses [7–9]. These analyses showed that the serological response to this highly complex virus spanned a large number of different proteins, interestingly also including a large number of nonenvelope proteins. Furthermore, very different patterns of binding were observed among the donors analyzed. A few major epitopes/proteins, however, did stand out by being recognized by almost all of the donors’ serum antibodies, for example, binding to H3 (an envelope protein) and A10 (a major core protein) were observed in all the donors analyzed. Importantly, the patterns observed in the vaccinated donors closely resembled those of serum samples isolated from smallpox convalescent donors, indicating that this approach would be a viable method to identify potential target proteins for vaccine design.
In addition to the serum antibody, the humoral immunity compartment consists of memory B cells and LLPCs. As serum antibody has a relatively short half-life [10, 11] it has to be continuously replenished by LLPCs that constitutively produce and secrete antibody. These terminally differentiated cells reside mainly in the bone marrow but are also found in secondary lymphoid tissue and in various inflamed tissues [12–18]. Plasma cells continue to secrete antibody in the absence of antigenic stimulation for extended periods of time but are unable to respond to further antigenic exposure. In contrast, memory B cells specific for a particular pathogen are normally present in a quiescent state but are able to respond to an antigenic challenge by vigorous expansion and the subsequent differentiation into additional antibody secreting cells. Memory B cells are not only present in relatively high numbers prior to exposure, but they are also primed to undergo very rapid expansion and differentiation. As such, these cells provide the reactive portion of the B cell-mediated immune memory. Similarly to plasma cells, memory B cells can also be sustained for very long periods of time. In the case of smallpox vaccination, memory B cells appear in blood around 14 days after vaccination (unpublished data). Cross-sectional studies showed that, while the number of memory B cells drop somewhat during the first year [3] postvaccination, they can be readily detected at stable levels for as many as up to 50 years after vaccination. Importantly, as smallpox has not been in circulation at least for the last 30 years, these experiments clearly showed that the memory B cells were maintained in the absence of antigenic stimulation [3, 4].
Maintenance of Humoral Immunity in Humans
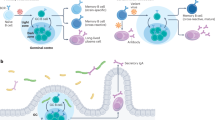
As mentioned above, LLPCs can sustain continual production of antibodies. However, it remains unclear how LLPCs specific to previously encountered antigens are maintained over very long periods of time, while also allowing LLPCs with novel specificities to take up residence in the bone marrow. The general belief is that several factors contribute to create so-called survival “niches” within the bone marrow microenvironment such that only a finite number of LLPC can be sustained. The bone marrow capacity in humans has been estimated to be about 1 × 109 LLPC, or on the order of 0.1–1% of the total bone marrow cells [17, 18]. Several mechanisms as to how this homoeostasis is maintained have been proposed from work both in humans and in mice. While early studies of plasma cells in secondary lymphoid organs during acute immune responses suggested that plasma cells had a very short half-life [19–23], more recent studies in mice showed that LLPCs can have exceptionally long life spans [13, 15, 24]. While longevity in itself could possibly explain long-term humoral immunity, deletion of all memory B cells by irradiation of immune mice did make the LLPC numbers slowly decline over time, with a half-life of about 140 days [15]. This suggested that memory B cells might have a function in replenishing LLPCs over time (Fig. 2). In the human case, where the LLPCs would have to be maintained for up to 60 years, the possibility of replenishment from the memory B cells pool seems plausible. Indeed, human studies did seem to support this possibility by showing that memory B cells can undergo nonantigen-specific activation (bystander activation) in vitro by activation through Toll-like reseptor ligands and/or bystander T-cell stimulation [25]. These studies also identified very low numbers of antibody-secreting cells in peripheral blood of vaccinated donors that were specific for antigens other than the immunizing one [25], raising the possibility that bystander activation of memory B cells could be a mechanism for maintenance of LLPC numbers. However, other studies have shown persistent increases only of antibody titers for the immunizing antigen after a booster vaccination with tetanus toxoid [26, 27]. Our own studies of antibody titers to various childhood vaccines after a primary vaccination with either the smallpox or yellow fever attenuated vaccines showed no detectable increases other than the antigen-specific ones, despite very large CD4 T cell responses and strong inflammatory signals (manuscript in preparation). This suggests that bystander activation as a mechanism for plasma cell maintenance may not have a physiologically relevant effect on serum antibody titers to other antigens, even in the presence of very strong inflammatory signals and plentiful CD4 T cell bystander help. Furthermore, if memory B cells indeed do sustain the numbers of LLPC over time, a correlation between the numbers of memory B cells and the antibody titers to a particular pathogen/vaccine would be expected. A recent study by Amanna and coworkers [1] demonstrated that, while some antigens did indeed show such a correlation, several did not, indicating that these two compartments may be independently regulated. Other studies in humans have proposed a “competitive dislocation” mechanism to explain the nonantigen-specific cells observed in peripheral blood after vaccination [28]. Here, a new immune response would dislocate preexisting LLPCs from their niches in the bone marrow to make room for the newly produced plasma cells. The issue of maintenance of humoral immunity remains to be fully understood and is the goal of continued studies to address this important issue.
Two models of antigen independent long-term maintenance of antibody titers. Model 1 shows replenishment of the LLPC pool by differentiation of memory B cells driven by one or many of the various suggested mechanisms. Model 2 depicts a scenario where the memory B cell pool and the LLPC pool are independently regulated. It is worth pointing out that these two models are not mutually exclusive
Vaccination with Live Attenuated Viruses Induces Large Effector T Cell Responses
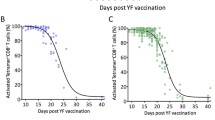
Our understanding of antiviral CD8 T cell responses in humans is largely based on studies of chronic viral infections. Thus, there is a need for further studies in humans to accurately characterize primary CD8 T cell responses to different acute viral infections. We have used the highly efficient smallpox and yellow fever vaccines to study the immune responses to acute infection. To identify expanding effector CD8+ T cells after immunization with live-attenuated YF-Vax or Dryvax smallpox vaccine, we monitored for the expression of CD38, HLA-DR, and Ki-67 and the down regulation of Bcl-2 on T cells in the blood. Differential preimmunization expression of these markers allowed for the unequivocal identification of the expanding populations of activated T cells, which was confirmed by functional analysis (IFN-γ production) and MHC class I tetramer staining. By analyzing HLA-DR and CD38 expressing CD3+ CD8+ lymphocytes at multiple time points postimmunization, we evaluated the magnitude and kinetics of the T cell responses after immunization. The peak of the CD8+ T cell response was observed at day 15 postimmunization, when 4–13% of peripheral CD8+ T cells coexpressed CD38 and HLA-DR after YF-Vax immunization and 10–40% of peripheral CD8+ T cells expressed an activated phenotype after smallpox immunization. Thus, immunization with the YF-Vax and Dryvax vaccines induced massive expansions of activated T cells. The number of activated CD8+ T cell declined after day 15, returning to baseline by day 30 postimmunization. Although the Dryvax immunization resulted in a three- to fourfold larger response than YF-Vax immunization, the kinetics of the YF-Vax- and Dryvax-induced responses were identical, supporting the idea that the T cell response may follow a distinct temporal course after an acute viral infection. We observed similar response kinetics for CD4+ T cells and neutralizing antibody titers. In contrast, T and B cell responses to chronic infections (such as human immunodeficiency virus, HBV, and hepatitis C virus) can take many weeks or months to develop after infection and are sustained for prolonged periods of time [29–31]. Thus, acute and chronic infections appear to induce very distinct response kinetics.
Minimal Role of Bystander CD8+ T Cell Proliferation After Acute Viral Infection
The role of bystander activation during antiviral T cell responses has been a long-standing and controversial issue, primarily studied in murine infection models. Previous studies suggested that nonspecific CD8 T cells could display an activated phenotype and perform cytolytic T cell functions in the absence of T cell receptor (TCR) engagement [32–34]. However, with the advent of MHC tetramer technology, it is now clear that large populations of effector CD8 T cells found in LCMV-infected mice and human patients with infectious mononucleosis are entirely antigen-specific [35]. These studies suggest that TCR engagement is the primary driving force behind the activation of anti-viral CD8 T cells, but they do not disprove a possible, albeit small, role for bystander activation.
To evaluate possible CD8+ T cell bystander activation during acute viral infection, we monitored the activation status of preexisting nonvaccine-specific memory CD8+ T cells [Epstein Barr virus (EBV), cytomegalovirus (CMV), and influenza] after immunization with live vaccines. Activation of these unrelated memory CD8+ T cells was evaluated after Dryvax and YF-Vax immunization by analyzing the expression patterns of activation markers on MHC class I tetramer+ T cells that were specific to the vaccine. We found that bystander CMV, EBV, and influenza-specific tetramer+ CD8+ T cells were neither activated nor proliferating in the presence of a strong vaccine-induced response. This reveals that bystander CD8+ T cell proliferation does not contribute to the peak of the CD8+ T cell response after immunization and that the observed effector responses appear antigen-specific. This implies that antiviral immune responses are highly specific and that memory CD8+ T cells are unlikely to be maintained by bystander activation.
Differentiation of Virus-specific CD8+ T Cells
We used an MHC class I tetramer [36] to further characterize the dynamics of the response and the phenotype of VV-specific effector and memory CD8+ T cells. At day 15 postimmunization, VV tetramer staining revealed a population of activated CD8+ T cells. Phenotypically, these cells were characterized by the up-regulation of CD38, CD27, HLA-DR, Ki-67, CCR5, perforin, and granzyme B and down-regulation of Bcl-2 and CCR7. These data support a model in which primed effector cells exit lymph nodes by down-regulating CCR7, migrate to infected tissues via CCR5, and kill infected cells by secreting perforin and granzyme. Further, expression of perforin and granzyme B, hallmarks of effector T cells, indicates that all tetramer+ CD8+ T cells pass through an obligate effector phase at the peak of the effector T cell response.
Analysis of VV-specific memory cells showed that most antigen-specific cells had undergone further differentiation and no longer exhibited an effector phenotype. Tetramer+ memory HLA-A2 VVCLT+ T cells were CD45RA+ and CD27+, had down-regulated CD38, HLA-DR, and Ki-67 and re-expressed Bcl-2 and CD127. Bimodal expression patterns were observed for CCR5, CCR7, CD62L, CD28, and Granzyme B. Memory CD8+ T cells had down-regulated PD-1 expression, consistent with their full functionality (Fig. 3). The bimodal expression patterns of granzyme B, CCR5, CCR7, CD62L, and CD28 suggest that memory T cell populations may be further subdivided based on their homing marker expression and cytotoxic potential.
Memory CD8 T cells specific for smallpox or yellow fever display a polyfunctional profile. Upon restimulation with antigen, specific CD8 T cells can kill infected cells, secrete cytokines, and expand exponentially. This is in contrast to memory cells specific for chronic viruses, which display various stages of functional exhaustion
Based on the distinct phenotypes of effector and memory CD8+ T cells, we asked whether the transition from an effector to a memory cell phenotype was an abrupt or gradual event, and whether this transition occurs simultaneously for different markers. To address this question, we analyzed the expression patterns of a selected set of markers (Ki-67, CD45RA, CCR5, and granzyme B) on tetramer+ CD8+ T cells at multiple time points after immunization (days 15, 21, 28, and 84). Interestingly, effector to memory transition occurred gradually over several weeks of time and did not progress simultaneously for all markers. For instance, Ki-67 down-regulation was an early event with most tetramer+ cells down-regulating Ki-67 expression by day 21 after immunization. In contrast, the change from CD45RO to CD45RA and the down-regulation of granzyme B occurred with slower kinetics. Combined, these data suggest that antigen-specific CD8+ T cells pass through several phenotypic stages after the peak of the effector response. However, the nonsimultaneous, gradual changes in the expression patterns of several key markers, suggest that effector to memory CD8+ T cell differentiation is a continuous and not a succession of discrete phenotypic stages. Because antigen-specific effector cells gradually differentiated into memory cells, these data support a linear differentiation process, where naive cells expand and differentiate into effector cells following antigen stimulation, then contract and slowly differentiate into a stable population of long-lived memory cells.
CD45RA+ tetramer+ CD8+ T cells were still detectable in the blood at low frequencies over 2 years postvaccination. Expression of CD45RA has been proposed as a surrogate marker for terminally differentiated memory CD8+ T cells, with limited proliferative potential [37]. By stimulating these populations with antigen directly ex vivo, we showed that these populations are not terminally differentiated and maintain polyfunctional T cell capabilities. Memory T cells stimulated with antigen secreted IL-2, TNF-α, MIP-1β, and IFN-γ, showing that they are capable of modulating the recall response. Upon stimulation, these cells also transiently expressed CD107A, a lysosomal membrane protein. Expression of CD107A on the cell’s surface indicates a cell has undergone recent cellular degranulation and, thus, has the capacity to kill infected cells. Finally, memory T cells also retained the ability to rapidly undergo multiple rounds of proliferation in response to antigen, a hallmark of memory cells. Extensive phenotypical analysis showed that memory CD8+ T cells exhibited multiple phenotypic profiles based on cytokine excretion (IL-2, TNF-α, MIP-1β, and IFN-γ) and expression of T cell markers (CCR7, CD62L, CCR5, Granzyme B, and CD28).
Concluding Remarks
In humans, antiviral T cell responses have been largely analyzed in the context of cross sectional studies of chronic infections, such as human immunodeficiency virus, CMV, hepatitis C virus, and EBV [38–47]. Based on these studies, it has become clear that the resulting populations of virus-specific T cells display striking phenotypic differences [37, 47–50], implying that viral persistence has a distinct impact on the character of the differentiation pathways of the adaptive immune response. To further explore the complex relationship between viral persistence and immune memory [48], our laboratory has analyzed the T and B cell responses induced after vaccination with the live viral yellow fever and smallpox vaccines in humans. Using these two attenuated viruses, we conducted multiple longitudinal studies to characterize the antiviral effector response and the formation and maintenance of immune memory in humans. We were able to detect VV-specific T cells, memory B cells, and antibody in individuals that were vaccinated more than 50 years prior. Given the long-term memory that results from YF-Vax and Dryvax immunization, the rapid kinetics we observed during the primary response may represent an optimal response, whereas responses that diverge from the above kinetics may be causally related to viral persistence. The transient viremia that occurs after vaccination implies that the transition from effector to memory cells occurs in the absence of antigenic stimulation. In contrast, the effector-to-memory transition during chronic infection is most likely associated with persistent antigenic stimulation. This difference in the persistence and degree of signaling may differentially affect the maturation pathway of responding cells during acute and chronic infections, resulting in distinct cellular phenotypes and functional capacities. Thus, persistent antigen exposure during the memory formation phase could lead to different, and possibly less functional, memory cells. This implies that vaccine boosting should occur after the formation of a phenotypically stable population of memory cells. Furthermore, drug therapies aimed at reducing viral loads during the early stages of chronic infections may result in the formation of more effective immune memory.
References
Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007;357:1903–15. doi:10.1056/NEJMoa066092.
Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev 2006;211:320–37. doi:10.1111/j.0105-2896.2006.00392.x.
Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 2003;171:4969–73.
Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med 2003;9:1131–7. doi:10.1038/nm917.
Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 1996;272:54–60. doi:10.1126/science.272.5258.54.
Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 2008;28:710–22. doi:10.1016/j.immuni.2008.02.020.
Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A 2005;102:547–52. doi:10.1073/pnas.0408782102.
Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol 2005;79:11724–33. doi:10.1128/JVI.79.18.11724-11733.2005.
Davies DH, Molina DM, Wrammert J, Miller J, Hirst S, Mu Y, Pablo J, Unal B, Nakajima-Sasaki R, Liang X, Crotty S, Karem KL, Damon IK, Ahmed R, Villarreal L, Felgner PL. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 2007;7:1678–86. doi:10.1002/pmic.200600926.
Talbot PJ, Buchmeier MJ. Catabolism of homologous murine monoclonal hybridoma IgG antibodies in mice. Immunology 1987;60:485–9.
Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 1988;18:313–6. doi:10.1002/eji.1830180221.
Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol 1998;10:1703–11. doi:10.1093/intimm/10.11.1703.
Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997;388:133–4. doi:10.1038/40540.
McMillan R, Longmire RL, Yelenosky R, Lang JE, Heath V, Craddock CG. Immunoglobulin synthesis by human lymphoid tissues: normal bone marrow as a major site of IgG production. J Immunol 1972;109:1386–94.
Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 1998;8:363–72. doi:10.1016/S1074-7613(00)80541-5.
Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol 2001;31:2726–32. doi:10.1002/1521-4141(200109)31:9<2726::AID-IMMU2726>3.0.CO;2-H.
Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 2006;6:741–50. doi:10.1038/nri1886.
Terstappen LW, Johnsen S, Segers-Nolten IM, Loken MR. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood 1990;76:1739–47.
Cooper EH. Production of lymphocytes and plasma cells in the rat following immunization with human serum albumin. Immunology 1961;4:219–31.
Levy M, Vieira P, Coutinho A, Freitas A. The majority of “natural” immunoglobulin-secreting cells are short-lived and the progeny of cycling lymphocytes. Eur J Immunol 1987;17:849–54. doi:10.1002/eji.1830170618.
Makela O, Nossal GJ. Autoradiographic studies on the immune response. II. DNA synthesis amongst single antibody-producing cells. J Exp Med 1962;115:231–44. doi:10.1084/jem.115.1.231.
Nossal GJ, Makela O. Autoradiographic studies on the immune response.I. The kinetics of plasma cell proliferation. J Exp Med 1962;115:209–30. doi:10.1084/jem.115.1.209.
Schooley JC. Autoradiographic observations of plasma cell formation. J Immunol 1961;86:331–7.
Slifka MK, Ahmed R. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol 1996;4:394–400. doi:10.1016/0966-842X(96)10059-7.
Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002;298:2199–202. doi:10.1126/science.1076071.
Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood 2006;107:2806–13. doi:10.1182/blood-2005-08-3255.
Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine 2001;20:498–504. doi:10.1016/S0264-410X(01)00328-0.
Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 2005;105:1614–21. doi:10.1182/blood-2004-07-2507.
Sakai A, Takikawa S, Thimme R, Meunier JC, Spangenberg HC, Govindarajan S, Farci P, Emerson SU, Chisari FV, Purcell RH, Bukh J. In vivo study of the HC-TN strain of hepatitis C virus recovered from a patient with fulminant hepatitis: RNA transcripts of a molecular clone (pHC-TN) are infectious in chimpanzees but not in Huh7.5 cells. J Virol 2007;81:7208–19. doi:10.1128/JVI.01774-06.
Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A 2002;99:15661–8. doi:10.1073/pnas.202608299.
Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68–76. doi:10.1128/JVI.77.1.68-76.2003.
Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 1996;272:1947–50. doi:10.1126/science.272.5270.1947.
Tough DF, Sprent J. Bystander stimulation of T cells in vivo by cytokines. Vet Immunol Immunopathol 1998;63:123–9. doi:10.1016/S0165-2427(98)00088-9.
Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med 1997;185:2089–94. doi:10.1084/jem.185.12.2089.
Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 1998;8:177–87. doi:10.1016/S1074-7613(00)80470-7.
Terajima M, Cruz J, Raines G, Kilpatrick ED, Kennedy JS, Rothman AL, Ennis FA. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J Exp Med 2003;197:927–32. doi:10.1084/jem.20022222.
Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001;410:106–11. doi:10.1038/35065118.
Barouch DH, Letvin NL. CD8+ cytotoxic T lymphocyte responses to lentiviruses and herpesviruses. Curr Opin Immunol 2001;13:479–82. doi:10.1016/S0952-7915(00)00244-2.
Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O'Callaghan CA, Steven N, McMichael AJ, Rickinson AB. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med 1998;187:1395–402. doi:10.1084/jem.187.9.1395.
Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997;186:1407–18. doi:10.1084/jem.186.9.1407.
Lauer GM, Ouchi K, Chung RT, Nguyen TN, Day CL, Purkis DR, Reiser M, Kim AY, Lucas M, Klenerman P, Walker BD. Comprehensive analysis of CD8(+)-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J Virol 2002;76:6104–13. doi:10.1128/JVI.76.12.6104-6113.2002.
Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med 2000;191:1499–512. doi:10.1084/jem.191.9.1499.
McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature 2001;410:980–7. doi:10.1038/35073658.
Roos MT, van Lier RA, Hamann D, Knol GJ, Verhoofstad I, van Baarle D, Miedema F, Schellekens PT. Changes in the composition of circulating CD8+ T cell subsets during acute epstein-barr and human immunodeficiency virus infections in humans. J Infect Dis 2000;182:451–8. doi:10.1086/315737.
Tan LC, Gudgeon N, Annels NE, Hansasuta P, O'Callaghan CA, Rowland-Jones S, McMichael AJ, Rickinson AB, Callan MF. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol 1999;162:1827–35.
Urbani S, Boni C, Missale G, Elia G, Cavallo C, Massari M, Raimondo G, Ferrari C. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J Virol 2002;76:12423–34. doi:10.1128/JVI.76.24.12423-12434.2002.
van Leeuwen EM, Gamadia LE, Baars PA, Remmerswaal EB, ten Berge IJ, van Lier RA. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol 2002;169:5838–43.
Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002;8:379–85. doi:10.1038/nm0402-379.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–12. doi:10.1038/44385.
Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol 2004;34:999–1010. doi:10.1002/eji.200324478.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wrammert, J., Miller, J., Akondy, R. et al. Human Immune Memory to Yellow Fever and Smallpox Vaccination. J Clin Immunol 29, 151–157 (2009). https://doi.org/10.1007/s10875-008-9267-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9267-3