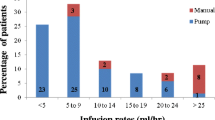
Subjects with primary immune deficiency diseases treated with intravenous immunoglobulin (n=42) received intravenous infusions of Carimune NF Liquid every 3–4 weeks for 6 months without routine premedication. The mean dose/patient/infusion was 278.5–800.7 mg/kg. Also, 80.4% of infusions achieved maximum rates of ≥3.5 mg/kg/min; 32% of infusions were associated with adverse events during or within 48 h of their end (upper 95% confidence interval was 39.4%, meeting the Food and Drug Administration (FDA) criterion for acceptable tolerability), and 54.8% of subjects had at least one temporally associated adverse event considered at least possibly drug-related (headache: 35.7% of subjects, 12.4% of infusions; nausea: 14.3%, 3.5%; myalgia: 14.3%, 3.2%; fatigue: 11.9%, 5.7%). The frequencies of these were highest after the first infusion. There were no serious drug-related adverse events or acute serious bacterial infections. Serum IgG trough levels were unchanged from baseline. Carimune NF Liquid, a ready-to-use, high-concentration, liquid immunoglobulin preparation is safe and effective.

Similar content being viewed by others
REFERENCES
Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, Kobrynski LJ, Levinson AI, Mazer B, Nelson RP Jr, Orange JS, Routes JM, Shearer WT, Sorensen RU: Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol 94:S1–S63, 2005 (available at: http://www.jcaai.org/pp/pp-immunodeficiency-update_05-0602.pdf)
Chapel HM: Consensus on diagnosis and management of primary antibody deficiencies. Consensus panel for the diagnosis and management of primary antibody deficiencies. BMJ 308:581–585, 1994
Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson RP Jr, Patel DD, Secord E, Sorensen RU, Wasserman RL, Cunningham-Rundles C: Primary immunodeficiency committee of the American Academy of Allergy, Asthma and Immunology: Use of intravenous immunoglobulin in human disease: A review of evidence by members of the primary immunodeficiency committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 117(suppl 4):S525–553, 2006
Roifman CM, Levison H, Gelfand EW: High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet 1:1075–1077, 1987
Durandy A, Wahn V, Petteway S, Gelfand E: Immunoglobulin replacement therapy in primary antibody deficiency diseases—maximizing success. Int Arch Allergy Immunol 136:217–229, 2005
Gelfand EW: Differences between IGIV products: Impact on clinical outcome. Int Immunopharmacol 6:592–599, 2006
Epstein JS, Zoon KC: Important drug warning: Immune globulin intravenous (human) (IGIV) products. Neonatal Netw 19:60–62, 2000
Centers for Disease Control and Prevention (CDC): Renal insufficiency and failure associated with immune globulin intravenous therapy—United States, 1985–1998. MMWR Morb Mortal Wkly Rep 48:518–521, 1999
Pierce LR, Jain N: Risks associated with the use of intravenous immunoglobulin. Transfus Med Rev 17:241–251, 2003
Borte M, Davies SV, Touraine J-L, Farber C-M, Lipsic T, Adams C, Spath P, Bolli R, Morell A, Andresen I: Clinical properties of a novel liquid intravenous immunoglobulin: Studies in patients with immune thrombocytopenic purpura and primary immunodeficiencies. Transfus Med Hemother 31:126–134, 2004
Andresen I, Schmugge M, Ridolfi-Lüthi A, Adams C, Bender K: An open label, multi center study in pediatric acute idiopathic thrombocytopenic purpura. Presented at the European Hematology Association Annual Congress, Lyon, 2003. Abstract number 0763 (http://eurocongres.com/eha2003/eha_scientific.html)
FDA: Draft guidance for industry. Safety, efficacy and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency. http://www.fda.gov/CBER/gdlns/igivimmuno.pdf
Roifman CM, Schroeder H, Berger M, Sorensen R, Ballow M, Buckley RH, Gewurz A, Korenblat P, Sussman G, Lemm G: Comparison of the efficacy of IGIV-C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Int Immunopharmacol 3:1325–1333, 2003
Bjorkander J, Nikoskelainen J, Leibl H, Lanbeck P, Wallvik J, Lumio JT, Braconier JH, Pavlova BG, Birthistle K, Engl W, Walter S, Ehrlich HJ: Prospective open-label study of pharmacokinetics, efficacy and safety of a new 10% liquid intravenous immunoglobulin in patients with hypo- or agammaglobulinemia. Vox Sang 90:286–293, 2006
Church JA, Leibl H, Stein MR, Melamed IR, Rubinstein A, Schneider LC, Wasserman RL, Pavlova BG, Birthistle K, Mancini M, Fritsch S, Patrone L, Moore-Perry K, Ehrlich HJ: US-PID-IGIV 10%-Study Group 10: Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin [IGIV 10%] in patients with primary immunodeficiency. J Clin Immunol 26:388–395, 2006
Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M: Subcutaneous IgG Study Group: Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol 26:265–273, 2006
Cassidy EA, Pinciaro PJ, Berger M: For the Grifols 0404-OLEIG201 investigators: Antibody deficiency in the new millennium: Illnesses, absences, and concomitant medications—100 patient years’ experience. J Allergy Clin Immunol 117:S110, 2006
Berger M, Pinciaro PJ: Flebogamma 5% investigators: Safety, efficacy, and pharmacokinetics of Flebogamma 5% [immune globulin intravenous (human)] for replacement therapy in primary immunodeficiency diseases. J Clin Immunol 24:389–396, 2004
Eijkhout HW, Van Der Meer JW, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA, Strengers PF, Nienhuis H, Schellekens PT: Inter-University Working Party for the Study of Immune Deficiencies: The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med 135:165–174, 2001
Nydegger UE, Sturzenegger M: Adverse effects of intravenous immunoglobulin therapy. Drug Safety 21:171–185, 1999
Ameratunga R, Sinclair J, Kolbe J: Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol 136:111–113, 2004
Work Loss Data Institute: Official disability guidelines. 6/17/02. Available at: http://www.disabilitydurations.com/pr_repmdc.htm, 2002
Gregori L, Maring JA, MacAuley C, Dunston B, Rentsch M, Kempf C, Rohwer RG: Partitioning of TSE infectivity during ethanol fractionation of human plasma. Biologicals 32:1–10, 2004
Siegel J: Safety considerations in IGIV utilization. Int Immunopharmacol 6:523–527, 2006
Shah S: Pharmacy considerations for the use of IGIV therapy. Am J Health Syst Pharm 62:S5–11, 2005
Orbach H, Katz U, Sherer Y, Shoenfeld Y: Intravenous immunoglobulin: Adverse effects and safe administration. Clin Rev Allergy Immunol 29:173–184, 2005
Berger M: Use of IGIV in Primary Immune Deficiency. Parts I and II. In UpToDate, Steihm ER, 2004. Updated, 2006
Kroez M, Kanzy EJ, Gronski P, Dickneite G: Hypotension with intravenous immunoglobulin therapy: Importance of pH and dimer formation. Biologicals 31:277–286, 2003
Bleeker WK, Teeling JL, Verhoeven AJ, Rigter GM, Agterberg J, Tool AT, Koenderman AH, Kuijpers TW, Hack CE: Vasoactive side effects of intravenous immunoglobulin preparations in a rat model and their treatment with recombinant platelet-activating factor acetylhydrolase. Blood 95:1856–1861, 2000
Spycher MO, Bolli R, Hodler G, Gennari K, Hubsch A, Späth P, Schnorf J: Well-tolerated liquid intravenous immunoglobulin G preparations (IGIVs) have a low immunoglobulin G dimer content. J Autoimmunity 96(suppl):96 (abstract), 1999
ACKNOWLEDGMENTS
This project was funded in part by grant MOI-RR02172 from the NCCR, NIH to the Children’s Hospital Boston GCRC, and this study was supported by CSL Behring. The authors would like to thank Dr. Jo Oswald and Dr. Catherine Jones, Watermeadow Medical, for their editorial assistance. Dr. Berger has received speaking and/or consultancy fees from CSL Behring, Talecris, INC Research, Grifols, Inc., FFF Enterprises and America’s Health Insurance Plans (contractor for Centers for Disease Control) and has received research funding from Talecris. Dr. Cunningham-Rundles has been a member of Medical Advisory Boards of Talecris and Omrix. Dr. Bonilla has received grant support from Talecris Biotherapeutics and is on the Medical Advisory Committee of the Immune Deficiency Foundation. Dr. Melamed has no relevant conflicts of interest. Dr. Bichler and Dr. Zenker are employees of CSL Behring. Dr. Ballow has been a speaker for and is a member of the Advisory Board of Talecris Biotherapeutics.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the study group
Rights and permissions
About this article
Cite this article
Berger, M., Cunningham-Rundles, C., Bonilla, F.A. et al. Carimune NF Liquid is a Safe and Effective Immunoglobulin Replacement Therapy in Patients with Primary Immunodeficiency Diseases. J Clin Immunol 27, 503–509 (2007). https://doi.org/10.1007/s10875-007-9096-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-007-9096-9