Abstract
The new noncentrosymmetric oxalate with the general formula [C6H16N2O]2(HC2O4)4·3H2O, has been synthesized and single crystals were grown from aqueous solution by slow evaporation technique. Single crystal X-ray diffraction study on grown crystal reveals that they belong to triclinic system with space group P1. The unit cell dimensions are: a = 5.688(2) Å, b = 8.315(2) Å, c = 16.634(3) Å, α = 96.63(2)°, β = 99.81(3)°, γ = 110.01(2)° with V = 715.4(3) Å3 and Z = 1. The structure has been solved using a direct method and refined to a reliability R factor of 0.052. The structure is built up from anionic chains parallel to the a-axis. These chains are interconnected by the organic cations and water molecules via hydrogen bonds so as to build a three dimensional arrangement. FT-IR spectrum confirms the presence of the functional groups in the synthesized material. The 13C CP-MAS NMR spectrum is in agreement with the X-ray structure. UV–Vis spectrum indicates that the crystal is transparent in UV and visible region with a lower cutoff wavelength of 212 nm. The DSC curve indicates that the crystal is thermally stable up to 330 K.
Graphical Abstract
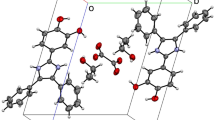
The molecular structure of bis(N-(2-hydroxyethyl)piperazine-1,4-diium) Tetrakis(hydrogen oxalate) Trihydrate is formed by N-(2-hydroxyethyl)piperazine-1,4-diium cations, oxalate anions and water molecules held together by means of N–H⋯O, C–H⋯O, O–H⋯OW and OW–H⋯OW(O) hydrogen bonds to form three-dimensional network.









Similar content being viewed by others
References
Dhanalakshmi B, Ponnusamy S, Muthamizhchelvan C (2010) J Cryst Growth 313:30
Chithambaram V, Das SJ, Krishnan S (2011) J Alloys Compd 509:4543–4546
Nalwa SH (1991) Appl Organomet Chem 5:343
Qin J, Su N, Dai C, Yang C, Liu D, Day MW, Wu B, Chen C (1999) Polyhedron 18:3461
Moerner WE, Silence SM (1994) Chem Rev 94:127
Vijayan N, Bhagavannarayana G, Maurya KK, Sharma SN, Gopalakrishnan R, Jayabharathi J, Ramasamy P (2012) Optik 123:604
Cohen MR, Hinsch E, Palkoski EZ, Vergona R, Urbano S, Sztokalo J (1982) J Pharmacol Exp Ther 223:110
Conrado DJ, Verli H, Neves G, Fraga CA, Barreiro EJ, Rates SM, Dalla-Costa T (2008) J Pharm Pharmacol 60:699
Neves G, Fenner R, Heckler AP, Viana AF, Tasso L, Menegatti R, Fraga CAM, Barreiro EJ, Dalla-Costa T, Rates SMK (2003) Braz J Med Biol Res 36:625
Sheldrick GM (1997) SHELXS97 and SHELXL97, Program for crystal structure solution and refinement. University of Gottingen, Göttingen
Farrugia LJ (1999) J Appl Cryst 32:837
Farrugia LJ (1997) J Appl Cryst 30:365
Newkome GR, Theriot KJ, Fronczek FR (1985) Acta Cryst C41:1642
Allen FH (2002) Acta Cryst B58:380
Derissen JL, Smith PH (1974) Acta Cryst B30:2240
Rong T (2011) Acta Cryst E67:m396
Hendrickson JB (1961) J Am Chem Soc 83:4537
Lett RG, Petrakis L, Ellis AF, Jensen RK (1970) J Phys Chem 74:2816
Tasal E, Kumalar M (2012) Spectrochim Acta 96:548
Petrosyan AM, Sukiasyan RP, Karapetyan HA, Antipin MY, Aprey RA (2005) J Cryst Growth 275:e1927
Silverstein M, Clayton Basseler G, Morill C (1981) Spectrometric identification of organic compound. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Essid, M., Rzaigui, M. & Marouani, H. Synthesis and Physico-Chemical Studies of a New Noncentrosymmetric Bis[N-(2-hydroxyethyl)piperazine-1,4-diium] Tetrakis(hydrogen oxalate) Trihydrate: [C6H16N2O]2(HC2O4)4·3H2O. J Chem Crystallogr 45, 310–317 (2015). https://doi.org/10.1007/s10870-015-0597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-015-0597-8