Abstract
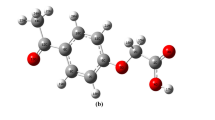
An X-ray diffraction study along with crystal supramolecular analysis of 3-(3-nitrothien-2-yl)indole, I, were carried out. The crystals are orthorhombic; C12H8N2O2S; M = 244.26; a = 13.3286 (6) Å, b = 7.5109 (4) Å, c = 21.8144 (9) Å; α = β = γ = 90°; V = 2183.83 (18) Å3, d c = 1.486 g/cm3, Z = 8, space group Pbca. The crystal structure of the title compound consists of two planar rings, the indole ring and the substituted thiophene ring. The inter-planar angle between the indole ring system and thiophene ring is 34.39°. The dihedral angle of the NO2 plane and the thiophene plane is 5.09°. The crystal packing shows crystal supramolecularity in which molecules are connected via different C–H···O, N–H···C(π) intermolecular interactions with edge-to-face and offset-face-to-face aryl···aryl, interactions consolidating three-dimensional network. The molecular geometry and vibrational frequencies of (C12H8N2O2S) in the ground state have been calculated by using density functional method (DFT/B3LYP) with 6-311++G(d,p) basis set. The optimized geometric bond lengths and bond angles and IR stretching frequencies obtained show good agreement with the experimental data.
Graphical Abstract
The crystal structure reveals different crystal supramolecularity motifs via C–H···O, N–H···C(π) and edge-to-face and offset-face-to-face aryl···aryl, interactions consolidating three-dimensional network. Density functional method (DFT/B3LYP) with 6-311++G(d,p) basis set of the obtained optimized geometric and IR stretching frequencies show good agreement with the experimental data.









Similar content being viewed by others
References
Kochanowska-Karamyan AJ, Hamann MT (2010) Chem Rev 110:4489
Barden TC (2011) Top Heterocycl Chem 26:31
Ali NAS, Dar BA, Pradhan V, Farooqui M (2013) Mini Rev Med Chem 13:1792
Abu Safieh KA, El-Abadelah MM, Zarga MHA, Sabri SS, Voelter W, MÖssmer CM (2001) J Heterocycl Chem 38:623
Moosa BA, Abu Safieh KA, El-Abadelah MM (2002) Heterocycles 57:1831
Agilent (2012) CrysAlis PRO. Agilent Technologies, Yarnton
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339
Sheldrick GM (2000) SHELXTL (version 6.10), Structure determination software suite
Frisch MJ et al (2003) Gaussian 03, Revision B.03. Pittsburgh PA, USA: Gaussian Inc.
Becke AD (1993) J Chem Phys 98:564
Lee C, Yang W, Parr RG (1988) Phys Rev B37:785
Reva I, Lopes Jesus AJ, Rosado MTS, Fausto R, Eusébio ME, Redinha JS (2006) Phys Chem Chem Phys 8:5339
Andrzejewska A, Lapinski L, Reva I, Fausto R (2002) Phys Chem Chem Phys 4:3289
Jaworskaa A, Maleka K, Marzecb KM, Baranska M (2012) Vib Spectrosc 63:469
Jamróz MH (2004) Vibrational energy distribution analysis VEDA 4. Warsaw
Beddoes RL, Dalton L, Joule TA, Mills OS, Street JD, Watt CIF (1986) J Chem Soc Perkin Trans 2:787
Paramasivam S, Purushothaman S, Seshadri PR, Raghunathan R (2013) Acta Cryst E69:o314
de Jager JJ, Smith VJ (2012) Acta Cryst E68:o3486
Ge Y-H, Han P, Wei P, Ou-yang P-K (2010) Acta Cryst E66:o2390
Majoube M, Vergoten G (1992) J Raman Spectrosc 23:431
Majoube M (1989) J Raman Spectrosc 20:49
Majoube M (1988) J Phys Chem 92:2407
Ozel AE, Gunduz SK, Celik S, Akyuz S (2013) J Spectrosc. doi:10.1155/2013/538917
Lautie MF (1978) Ph.D thesis, Universitb Pierre et Marie Curie, Paris
Lautie A, Lautie MF, Gruger A, Fakhri SA (1980) Spectrochim Acta Part A 36:85
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu-Safieh, K.A., Khanfar, M.A., El-Barghouthi, M.I. et al. Molecular Structure and Density Functional Theory Calculations of 3-(3-Nitrothien-2-yl)indole: Structural and Vibrational Analysis. J Chem Crystallogr 44, 330–336 (2014). https://doi.org/10.1007/s10870-014-0519-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-014-0519-1