Abstract
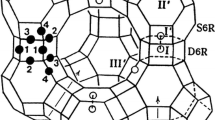
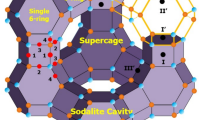
The single-crystal structure of fully dehydrated zeolite Y (FAU, Si/Al = 1.56) Mn2+ exchanged by flow method, |Mn31(Mn4AlO4)H10|[Si117Al75O384]-FAU, was determined by single-crystal synchrotron X-ray diffraction techniques. It was refined to the final error indices R 1/wR 2 = 0.0692/0.2026 in the cubic space group \(Fd\overline{3}m\) at 100(1) K. In this structure, 35 Mn2+ ions per unit cell are found at four crystallographic positions: 11 and 7 are at the centers of the double 6-rings (site I) and in the sodalite cavity opposite the double 6-rings (site I′), respectively. The remaining 4 and 13 are found at sites II′ (near 6-ring in the sodalite cavity) and II (near 6-ring in the supercage), respectively. Some dealumination of the zeolite framework occurred during Mn2+ exchange. The four non-framework oxygen atoms coordinate to a aluminate ion at the center of sodalite cavity (site U) and 4 Mn2+ ions at site II′ to give Mn4AlO4 3+. The 10 H+ ions are required for charge balance in fully dehydrated Mn2+-Y.
Graphical Abstract
The single-crystal structure of fully dehydrated, partially dealuminated, and Mn2+-exchanged zeolite Y (FAU (Si/Al = 1.56) was determined by single-crystal synchrotron X-ray diffraction techniques.





Similar content being viewed by others
References
Seo SM, Lim WT, Seff K (2013) Microporous Mesoporous Mater 170:67–74
Bae D, Seff K (1999) Microporous Mesoporous Mater 33:265–280
Einaga H, Futamura S (2007) Catal Commun 8:557–560
Einaga H, Teraoka Y, Ogat A (2011) Catal Today 164:571–574
Aboul-Gheit A, Ahmed SM, Hanafy SA (2008) J Mol Catal A 288:52–57
Qi B, Lu X-H, Zhou D, Xia Q-H, Tang Z-R, Fang S-Y, Pang T, Dong Y-L (2010) J Mol Catal A 322:73–79
Meyer CI, Borgna A, Monzón A, Garetto TF (2011) J Hazard Mater 190:903–908
Regenhardt SA, Meyer CI, Trasarti AF, Monzón A, Garetto TF (2012) Chem Eng J 198–199:18–26
Jang SB, Jeong MS, Kim Y, Seff K (1997) J Phys Chem B 101:9041–9045
Seo SM, Lim WT (2010) Bull Korean Chem Soc 31:2379–2382
Lim WT, Seo SM, Wang L, Lu GQ, Heo NH, Seff K (2010) Microporous Mesoporous Mater 129:11–21
Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–326
Bruker-AXS (ver. 6.12) (2001) XPREP program for the automatic space group determination. Bruker AXS Inc., Madison
Sheldrick GM (1997) SHELXL97. Program for the refinement of crystal structures. University of Gottingen, Germany
Doyle PA, Turner PS (1968) Acta Crystallogr Sect A 24:390–397
Ibers JA, Hamilton WC (1974) International tables for X-ray crystallography, vol IV. Kynoch Press, Birmingham, pp 71–98
Cromer DT (1965) Acta Crystallogr 18:17–23
Ibers JA, Hamilton WC (1974) International tables for X-ray crystallography, vol IV. Kynoch Press, Birmingham, pp 148–150
Breck DW (1974) Zeolite molecular sieves. Wiley, New York, pp 93–103
Van Bekkum H, Flanigen EM, Jacobs PA, Jansen JC (2001) Introduction to zeolite science and practice. Elsevier, New York, p 44
Weast RC (ed) (1989/1990) Hand book of chemistry and physics, 70th edn. CRC Press, Cleveland, p F-187
Shamsuzzoha Md, Seo SM, Kim YH, Lim WT (2011) J Incl Phenom Macrocycl Chem 70:59–68
Shamsuzzoha Md, Kim YH, Lim WT (2011) J Phys Chem C 115:24681–24687
Shamsuzzoha Md, Kim YH, Lim WT (2011) J Phys Chem C 115:17750–17760
Shamsuzzoha Md, Seo SM, Kim YH, Lim WT (2011) Microporous Mesoporous Mater 143:326–332
Bae MN, Kim Y, Seff K (1998) Microporous Mesoporous Mater 26:101–107
Bae MN, Kim Y (1998) Bull Korean Chem Soc 19:1095–1099
Choi EY, Kim Y, Han YW, Seff K (2000) Microporous Mesoporous Mater 40:247–255
Kim Y, Kim AN, Han YW, Seff K (1999) Proceedings of the 12th international zeolite conference, vol IV. Materials Research Society, Warrendale, PA, pp 2839–2846
Jeong GH, Kim Y, Seff K (2006) Microporous Mesoporous Mater 93:12–22
Bae MN, Song MK, Kim Y, Seff K (2003) Microporous Mesoporous Mater 63:21–31
Seo SM, Lim WT, Seff K (2012) J Phys Chem C 116(16):13985–13996
Bae D, Seff K (2000) Microporous Mesoporous Mater 40:219–232
Kim CW, Jung KJ, Heo NH, Kim SH, Hong SB, Seff K (2009) J Phys Chem C 113:5164–5181
Seo SM, Lim WT, Seff K (2012) J Phys Chem C 116(1):963–974
Loewenstein W (1954) Am Mineral 39:92–96
Acknowledgments
The authors wish to thank the staff at beamline 6B MXI of the Pohang Light Source, Korea, for assistance during data collection. This work was supported by a special Grant (contract number: 2013–0173) from the Research Fund at Andong National University. This study was financially supported by the “2013 Post-Doc. Development Program” of Pusan National University.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, S.M., Suh, J.M. & Lim, W.T. Single-Crystal Structure of Fully Dehydrated and Partially Dealuminated Zeolite Y (FAU, Si/Al = 1.56) Mn2+ Exchanged by Flow Method. J Chem Crystallogr 44, 89–97 (2014). https://doi.org/10.1007/s10870-013-0487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0487-x