Abstract
A single crystal of fully dehydrated, largely Rb+-exchanged zeolite Y, |Rb59Na16|[Si117Al75O384]-FAU (Si/Al = 1.56), prepared by exchange of |Na75|[Si117Al75O384]-FAU with an aqueous stream 0.1 M RbOH at 293 K, followed by vacuum dehydration at 673 K and at 1 × 10−6 Torr. Its crystal structure was determined by single-crystal synchrotron X-ray diffraction techniques in the cubic space group Fd \(\overline{3}\) m and was refined to the final error indices R 1/wR 2 = 0.0392/0.1189. In the structure of |Rb59Na16|[Si117Al75O384]-FAU, about 59 Rb+ ions per unit cell occupy five different equipoints; 9 are at site I, 9 at site I′, 3 at site II′, 28 at site II, and the remaining 10 are at site III, preferring II. The residual 16 Na+ ions occupy three equipoints; 5 are at site I′, 3 at site II, and 8 are at site III. These Rb+ and Na+ ions filled full from hexagonal prism to supercage. This work achieved the highest level of Rb+-exchange (ca. 81 %) in the single-crystal structure of zeolite Y by conventional method using aqueous solution at room temperature. The distributions of Rb+ and Na+ ions in this structure, as compared to fully dehydrated partially Rb+-exchanged zeolite X, are different due to local Si/Al order among the T atoms.
Graphical Abstract
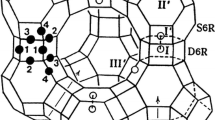
The single-crystal structure of fully dehydrated, largely Rb+-exchanged zeolite Y, |Rb59Na16|[Si117Al75O384]-FAU (Si/Al = 1.56), was determined by single-crystal synchrotron X-ray diffraction techniques.





Similar content being viewed by others
References
Lim WT, Seo SM, Wang L, Lu GQ, Seff K (2010) Microporous Mesoporous Mater 129:11–21
Lim WT, Seo SM, Kim GH, Lee HS, Seff K (2007) J Phys Chem C 111:18294–18306
Kim HS, Bae D, Lim WT, Seff K (2012) J Phys Chem C 116:9009–9018
Seo SM, Kim GH, Lee HS, Ko SO, Lee OS, Kim YH, Kim SH, Heo NH, Lim WT (2006) Anal Sci 22:x209–x210
Su H, Kim HS, Seo SM, Ko SO, Suh JM, Kim GH, Lim WT (2012) Bull Korean Chem Soc 33:2785–2788
Seo SM, Lee OS, Kim HS, Bae D, Chun IJ, Lim WT (2007) Bull Korean Chem Soc 28:1675–1682
Seo SM, Kim GH, Lee SH, Bae JS, Lim WT (2009) Bull Korean Chem Soc 30:1285–1292
Breck DW (1974) Zeolite molecular sieves. Wiley, New York, pp 92–107
Ziolek M, Czyzniewska J, Lamotte J, Lavalley JC (1996) Catal Lett 37:223–227
Ziolek M, Czyzniewska J, Kujawa J, Travert A, Mauge F, Lavalley JC (1998) Microporous Mesoporous Mater 23:45–54
Nam SS, Kim H, Kishan G, Choi MJ, Lee KW (1999) Appl Catal A Gen 179:155–163
Kirschhock CEA, Hunger B, Martens J, Jacobs PA (2000) J Phys Chem B 104:439–448
Marra GL, Fitch AN, Zecchina A, Ricchiardi G, Salvalaggio M, Bordiga S, Lamberti C (1997) J Phys Chem B 101:10653–10660
Shepelev YF, Butikova IK, Smolin YI (1991) Zeolites 11:287–292
Lee SH, Kim Y, Kim DS, Seff K (1998) Bull Korean Chem Soc 19:98–103
Lee SH, Kim Y, Seff K (2000) J Phys Chem B 104:11162–11167
Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–326
Bruker-AXS (ver. 6.12) (2001) XPREP. Program for the automatic space group determination. Bruker AXS Inc., Madison, Wisconsin, USA
Sheldrick GM (1997) SHELXL97. Program for the refinement of crystal structures. University of Gottingen, Germany
Doyle PA, Turner PS (1968) Acta Crystallogr Sect A 24:390–397
Ibers JA, Hamilton WC (1974) International tables for X-ray crystallography, vol IV. Kynoch Press, Birmingham, pp 71–98
Cromer DT (1965) Acta Crystallogr 18:17–23
Ibers JA, Hamilton WC (1974) International tables for X-ray crystallography, vol IV. Kynoch Press, Birmingham, pp 148–150
Loewenstein W (1954) Am Miner 39:92–96
Breck DW (1974) Zeolite molecular sieves. Wiley, New York, pp 93–103
Van Bekkum H, Flanigen EM, Jacobs PA, Jansen JC (2001) Introduction to zeolite science and practice. Elsevier, New York, p 44
Weast RC (ed) (1989/1990) CRC Press Handbook of chemistry and physics, 70th edn. CRC Press, Cleveland, OH, p F-187
Acknowledgments
The authors wish to thank the staff at Beamline 4A MXW at the Pohang Light Source, Korea, for assistance during data collection. This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MEST) (2013M2A8A5025633).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, S.M., Chun, E.Y., Chung, D.Y. et al. Single-Crystal Structure of Fully Dehydrated, Largely Rb+-Exchanged Zeolite Y (FAU, Si/Al = 1.56), |Rb59Na16|[Si117Al75O384]-FAU. J Chem Crystallogr 43, 401–408 (2013). https://doi.org/10.1007/s10870-013-0435-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0435-9