Abstract
Mononuclear molybdenum(V) starting material, (PyH)5[MoOCl4(H2O)]3Cl2 (where PyH+ stands for pyridinium cation, C5H5NH+), reacted with triethylammonium or pyridinium salt of pivalic acid (dimethylpropanoic acid) to form two dinuclear molybdenum(V) compounds with the (PyH)2[Mo2O4Cl3(Py)(Piv)] (1) and (PyH)[Mo2O4Cl2(Py)2(Piv)]·CH3CN (2) (Py = pyridine, Piv− = a deprotonated form of dimethylpropanoic acid) compositions. Compound 1 crystallized in the triclinic \( P{\bar1}\) space group with a = 9.52110(10) Å, b = 10.89790(10) Å, c = 14.5665(2) Å, α = 109.6310(6)°, β = 93.2646(7)° and γ = 111.8670(7)°. Compound 2 crystallized in the orthorhombic P nam space group with a = 10.67530(10) Å, b = 12.7801(2) Å and c = 20.1379(3) Å. X-ray structural analysis confirmed that the anions of both products consist of the central {Mo2O4}2+ core with the pivalato ligands coordinated to the sites which are trans to the terminal oxides. Because of a different pyridine/chloride content in the complex anions of 1 and 2, the metal–metal bond lengths also differ.
Graphical Abstract
Reaction of mononuclear molybdenum(V) starting material with pivalate resulted in (PyH)2[Mo2O4Cl3(Py)(Piv)] (1) and (PyH)[Mo2O4Cl2(Py)2(Piv)]·CH3CN (2) with the carboxylate coordinated as a bridging ligand to a pair of metal ions of the {Mo2O4}2+ core.






Similar content being viewed by others
Notes
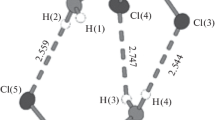
The contacts are slightly longer than the sum of the van der Waals radii of carbon and chlorine, 3.45 Å [22]. The C–H···Cl contacts are C21···Cl1 = 3.444(2), C25···Cl2 = 3.527(2) and C35···Cl3 = 3.550(2) Å in compound 1 and C21···Cl1 [x–0.5, –y + 1.5, z] = 3.520(2) Å in compound 2
References
Stiefel EI (1977) Prog Inorg Chem 1:1–223
Chae HK, Klemperer WG, Marquart TA (1993) Coord Chem Rev 128:209–224
Modec B, Brenčič JV (2002) J Clust Sci 13:279–302
Dinoi C, da Guedes Silva MFC, Alegria ECBA, Smolenski P, Martins LMDRS, Poli R, Pombeiro AJL (2010) Eur J Inorg Chem 16:2415–2424
Paredes P, Lopez-Calzada A, Miguel D, Villafane F (2010) Dalton Trans 39:10099–10104
Sreehari S, Luck RL (2010) J Clust Sci 21:525–541
Maass JS, Zeller M, Luck RL (2012) J Clust Sci 23:713–726
Mundi LA, Haushalter RC (1990) Inorg Chem 29:2879–2881
Modec B, Brenčič JV, Dolenc D, Zubieta J (2002) J Chem Soc Dalton Trans 4582–4586
Modec B, Dolenc D, Brenčič JV (2007) Inorg Chim Acta 360:663–678
Modec B (2008) Inorg Chim Acta 361:2863–2870
Modec B, Brenčič JV (2005) Eur J Inorg Chem 2005:1698–1709
Nakamoto K (1997) In: Infrared and Raman spectra of inorganic and coordination compounds. Part B: Applications in coordination, organometallic, and bioinorganic chemistry, 5th edn. Wiley, New York
Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–326
Sheldrick GM (1997) SHELXS-97 and SHELXL-97. University of Göttingen, Göttingen
Farrugia LJ (1997) J Appl Crystallogr 30:565
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) J Appl Cryst 39:453–457
Glowiak T, Sabat M, Sabat H, Rudolf MF (1975) J Chem Soc Chem Commun 712
Liu G, Liu J, Wei YG, Liu Q, Zhang SW (2000) Acta Cryst C56:822–823
Cindrić M, Strukan N, Kajfež T, Giester G, Kamenar B (2000) Inorg Chem Commun 3:281–284
Shibahara T, Kuroya H, Matsumoto K, Ooi S (1981) Inorg Chim Acta 54:L75–L76
Douglas B, McDaniel D, Alexander J (1994) In: Concepts and models of inorganic chemistry, 3rd edn. John Wiley & Sons, Inc., New York
Janiak C (2000) Dalton Trans 21:3885–3896
Acknowledgments
This work was supported by a Grant from the Slovenian Ministry of Education, Science and Sport (Grant P1-0134).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modec, B. Carboxylato Molybdenum(V) Complexes: X-Ray Structures of the Dinuclear Pivalato Complexes. J Chem Crystallogr 43, 377–382 (2013). https://doi.org/10.1007/s10870-013-0431-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0431-0