Abstract
The organic–inorganic hybrid material, [Na+(ClO4)−(C6H5NO3)(H2O)] n crystallizes in the triclinic space group P-1 with the unit-cell parameters a = 6.1756(3) Å, b = 7.3500(3) Å, c = 12.1415(6) Å, α = 100.386(4)°, β = 104.707(4)°, and γ = 97.069(4)°. In the crystal structure the picolinic acid N-oxide exists as a neutral molecule with very strong intramolecular O···H···O hydrogen bond between the carboxyl and N-oxide oxygen atoms. The O···O distance is 2.401(2) Å and the difference Fourier map shows clearly the position of the hydrogen atom approximately in the middle point between the oxygen atoms. The sodium atoms (which occupy two different centres of symmetry) are joined by water, perchlorate anion, and the carbonyl oxygen atom from picolinic acid N-oxide into 1D polymeric chain which extends along [100] direction. The neighbouring chains are connected by O(water)–H···O(perchlorate or picolinic acid N-oxide) hydrogen bonds into layers, which in turn interact with each other by means of short π···π dimeric interactions. The distance between the centroids of pyridine rings is 3.444 Å and the interplanar separation is only 3.195 Å.
Graphical Abstract
The sodium cations are joined by water, perchlorate anions, and the carbonyl oxygen atoms from picolinic acid N-oxide into 1D polymeric chain which extends along [100] direction.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Results and Discussion
After Janiak [1], the coordination polymer (or metal–organic coordination network) can be defined as “metal–ligand compound that extends infinitely via more or less covalent metal–ligand bonding”, and additionally at least one carbon atom has to lie between the donor atoms. If this last condition is not fulfilled it is more appropriate to use the term “organic–inorganic hybrid material” [2]. These materials received significant attention due to their diverse topologies and possible properties.
During our studies on structural and spectral properties of pyridine carboxylic acid N-oxides complexes with lanthanides [3] or hexavalent uranium [4, 5] we have determined the structures of a series of crystals in which these acids were deprotonated and formed anions. As a by-product of these studies the sodium complex (1, Fig. 1) has been obtained, in which the Na+ cations are coordinated by perchlorate anion, water and neutral picolinic acid N-oxide. In this latter molecule the potential closeness of OH and N → O groups (assuming non-typical antiplanar conformation of OH group) allows for formation of very strong intramolecular hydrogen bonds (according, e.g., to the classification by Jeffrey [6] and Steiner [7]), and indeed such bonds have been reported in similar molecules, for instance in picolinic acid N-oxide itself O···O distance is 2.425(2) Å [8], in quinaldic acid N-oxide—2.435(2) Å [9], in 6-methyl-picolinic acid N-oxide [10], 2.41 Å and in 2-carboxylic acid-4-nitropyridine N-oxide [10]: 2.460(2) Å.
In the Cambridge Crystallographic Database (version 5.33, Nov 2011 [11]) there are some examples of 1D polymeric structures involving sodium cations, for instance ditetrazole-tetra-water sodium coordination polymer [12] or catena-poly{[(2-methylbenzoato-κ2 O,O′)sodium]-di-μ-aqua-κ4 O:O′} [13]; however, we did not find an example of two different octagonal sodium cations in one polymeric chain.
In the crystal structure of 1 the picolinic acid N-oxide exists in the neutral form and there is a strong intramolecular hydrogen bond between carboxylic O21 and N-oxide O1 atoms (which closes almost planar six-membered ring), accompanied by the transfer of electron density towards the middle point of the bond. Figure 2 shows the residual electron density in this region after removing the H atom. The diffuse but well-defined electron density (max. of ca. 0.45 eÅ−3) is clearly seen between the oxygen atoms. The geometrical features of this hydrogen bond fulfill the conditions postulated for very strong hydrogen bonds [6, 7]. The O···O distance is short [2.401(2) Å], O–H bond is significantly lengthened [1.18(3) Å] and comparable with H···O distance [1.26(3) Å], and the bond is highly directional, especially taking into account that it is an intramolecular one. The transfer of electron density might also explain relatively short C21–O21 bond [1.278(2) Å] and long N–O one, of 1.3544(19) Å, which would be unexpected without involvement of the N-oxide group in either coordination or strong hydrogen bonding (cf. [14]). The participation of the carboxyl O–H bond in the intramolecular hydrogen bond causes also the antiplanar conformation of the carboxyl group (torsion angle C2–C21–O21–H21 is 3.0(16) °). This conformation is far less stable than the synplanar one and it occurs almost exclusively in molecules in which it is forced by an intramolecular hydrogen bond [15].
The residual electron density map of the O···O fragment of picolinic acid N-oxide residue in 1 without the hydrogen atom [15]. Contour level 0.1 eÅ−3. Negative blue lines, positive dashed red lines (Color figure online)
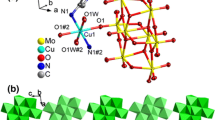
The crystal structure of 1 can be regarded as the result of the hierarchical sequence of interactions. Figure 3 shows the perspective view of the 1D polymer—first level—which expands along [010] direction. There are two symmetry-independent sodium cations, that occupy two different symmetry centers at (1/2, 0, 1/2) and (1/2, 1/2, 1/2). Each of them is coordinated, in a quite regular octahedral geometry, cf. Table 1, by six oxygen atoms: two from water molecules, two from picolinic acid N-oxide (O22), and two from perchlorate anion (O2A and O3A), which balances the total charge.
The fragment of the 1D polymer 1 [14]. Symmetry codes: (i) x, 1 + y, z; (ii) x, −1 + y, z
The Na–O bond distances are quite diverse, probably reflecting the different degree of covalent and ionic components. The CCD analysis shows, however, that the distribution of Na–O distances found in crystal structures implies different bonding interactions in the distance range (Fig. 4) 2.2–2.7 Å.
CSD distribution of Na–O distances [11]. The graph shows the number of hits for any kind of Na…O contacts in almost 5,000 crystal structures
The neighbouring polymeric chains are connected by means of O–H(water)…O hydrogen bonds—the second level—in which one of perchlorate oxygen atoms and picolinic acid N-oxide O21 atom act as acceptors (Table 2). These hydrogen bonds connect chains into 2D layer in the xy plane (Fig. 5), with pyridine rings sticking outward. Using the graph-set notation (cf. [16], for instance) one could find the different motifs created by the same hydrogen bonds: first-rank motifs could be identified as either C(6) chains or R 22 (14) rings; at the second level the R 22 (10) rings can be identified, in which both types of hydrogen bonds are involved.
Hydrogen bonded pair of the polymeric chains of 1, as seen approximately along [001] direction [14]. Hydrogen bonds are shown as dashed lines
Finally, at the third level, the pairs of these rings related by the centers of symmetry at (1/2, 0, 0) are connected by means of quite short π…π interactions. Janiak [17] classified the nitrogen-containing rings and found out that strong interactions exhibit rather short centroid–centroid contacts, shorter than 3.8 Å, small slip angles (<25°) and vertical displacements (<1.5 Å). In 1 the centroid-to-centroid distance is 3.444 Å, and the angle between the plane of the ring and the centroid–centroid line is 21.9°. That means that mean interplanar distance is as short as 3.195 Å with the vertical offset of 1.285 Å (Fig. 5).
Experimental
The sodium complex was prepared by serendipity during our studies on the complexes of lanthanides with the nicotinic acids. The detailed procedure is described in, colourless crystals of 1 were found as accompanying the Gd-complex after slow evaporation from water solution [3] (Fig. 6).
The packing of 1 as seen approximately along [100] direction [14]. Hydrogen bonds are drawn as dashed lines, the pairs of π…π interacting pyridine rings are seen in the central part of the diagram
X-ray diffraction data were collected at room temperature by the ω-scan technique, on an Agilent Xcalibur four-circle diffractometer equipped with sapphire CCD-detector using graphite-monochromatized MoKα radiation (λ-0.71073 Å) [18]. The data were corrected for Lorentz-polarization effects as well as for absorption [18]. Accurate unit-cell parameters were determined by a least-squares fit of 2,206 reflections of highest intensity, chosen from the whole experiment. The structures were solved with SIR92 [19] and refined with the full-matrix least-squares procedure on F 2 by SHELXL97 [20]. Most calculations were performed within WinGX suite [21]. Scattering factors incorporated in SHELXL97 were used. The function \( \sum w \left( {\left| {F_{0} } \right|^{2} - \left| {F_{\text{c}} } \right|^{2} } \right)^{2} \) was minimized, with \( w^{ - 1} = \left[ {\sigma^{ 2} \left( {F_{\text{o}} } \right)^{ 2} + \left( {0.0403 \cdot P} \right)^{2} + 0. 3 40 6 { }P} \right] \), where \( P = {{\left[ {{\text{Max}}.\;\left( {F_{\text{o}}{}^{2} ,\;0} \right) + 2F_{\text{c}}{}^{ 2} } \right]} \mathord{\left/ {\vphantom {{\left[ {{\text{Max}}.\;\left( {F_{\text{o}}{}^{2} ,\;0} \right) + 2F_{\text{c}}{}^{ 2} } \right]} 3}} \right. \kern-\nulldelimiterspace} 3} \). All non-hydrogen atoms were refined anisotropically; all hydrogen atoms were found in difference Fourier maps and isotropically refined. Relevant crystal data are listed in Table 3, together with refinement details.
Crystallographic data (excluding structure factors) for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, no. CCDC 858635. Copies of this information may be obtained free of charge from: The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK. Fax: +44(1223)336-033, e-mail: deposit@ccdc.cam.ac.uk, or www: www.ccdc.cam.ac.uk.
References
Janiak C (2003) Dalton Trans 2781
Hagrman PJ, Hagrman D, Zubieta J (1999) Angew Chem Int Ed Engl 38:2638
Lis S, Piskuła Z, Kubicki M (2009) Mater Chem Phys 114:134
Lis S, Glatty Z, Meinrath G, Kubicki M (2010) J Chem Cryst 40:646
Lis S, Meinrath G, Glatty Z, Kubicki M (2010) Inorg Chim Acta 363:3847
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Steiner T (2002) Angew Chem Int Ed Engl 41:48
Steiner T, Schreurs AMM, Lutz M, Kroon J (2000) Acta Cryst C56:577
Dideberg O, Dupont L (1975) Acta Cryst B31:2719
Wu W, Wu D, Cheng W, Zhang H, Dai J (2007) Cryst Growth Des 7:2316
Allen FH (2002) Acta Crystallogr B58:380
Huang X-F, Song Y-M, Wu Q, Ye Q, Chen X-B, Xiong R-G, You X-Z (2005) Inorg Chem Commun 8:58
Danish M, Saleem I, Ahmad N, Raza AR, Starosta W, Leciejewicz J (2010) Acta Cryst E66:m459
Eichhorn K (1987) Acta Cryst B43:111
Bernstein J, Etter MC, Leiserowitz L (1994) In: Bürgi H-B, Dunitz JD (eds) Structure correlation, vol 2. VCH, Weinheim, pp 431–507
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34:1555
Janiak C (2000) J Chem Soc Dalton Trans 3885
Agilent technologies (2011) CrysAlis PRO (Version 1.171.35.4)
Altomare A, Cascarano G, Giacovazzo C, Gualardi A (1993) J Appl Cryst 26:343
Sheldrick GM (2008) Acta Cryst A64:112
Farrugia LJ (1999) J Appl Cryst 32:837
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Piskuła, Z., Lis, S. & Kubicki, M. Organic–Inorganic Hybrid Material [Na+(ClO4)−(C6H5NO3)(H2O)]: Very Short Intramolecular Hydrogen Bond And Hierarchy of Intermolecular Interactions. J Chem Crystallogr 42, 588–592 (2012). https://doi.org/10.1007/s10870-012-0285-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0285-x