Abstract
The new cobalt compound, [Co(C16H35N10]+(NO3)−·CH3OH (1), have been synthesized and characterized. It’s structure and electrochemical property were investigated using single-crystal X-ray diffraction and cyclic voltammetry in solutions of dimethyl sulfoxide (DMSO) and methanol (CH3OH). The X-ray diffraction analysis reveals that 1 crystallizes in the monoclinic space group P21/c with a = 9.8339 (10) Å, b = 16.4048 (17) Å, c = 15.3628 (16) Å, β = 104.96 (9)° and Z = 4. The complex 1 consists of one [Co(C16H35N10)]+ cation, one NO3 − anions and one solvent CH3OH molecule. The metal center of complex 1 is six-coordinated, with the four nitrogens of the macrocycle defining an equatorial plane and two axially ligated [N3]− ions completing the cobalt coordination sphere. Moreover, hydrogen bonds of O–H⋯N, O–H⋯O and N–H⋯O and static force connect two components of the compound and maintain the crystal structure. Additionally, in situ spectro-electrochemical measurements support Co(III)/Co(II) reversible reduction processes with the observation of the corresponding spectral changes with the applied potentials E app = −1.5 and 0.5 V. The results obtained by the method are in agreement with those by the CV methods.
Graphical Abstract
The paper is about synthesis, crystal structure and electrochemical property of a new complex.

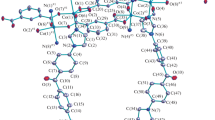
The molecular structure of the title compound (I) showing the atom-labeling scheme and displacement ellipsoids drawn at the 20% probability level



Similar content being viewed by others
References
Lehn J-M (1995) Supramolecular chemistry. VCH, Weinheim
Whitesides GM, Mathias JP, Seto CT (1991) Science 254:1275
Rebek J (1990) Angew Chem Int Ed Engl 29:102
LeCours SM, Guan H-W, DiMagno SG, Wang CH, Therien MJ (1996) J Am Chem Soc 118:1497
Darling SL, Stulz E, Feeder N, Bampos N, Sanders JKM (2000) New J Chem 24:261
Brandon EJ, Rogers RD, Burkhart BM, Miller JS (1998) Chem Eur J 4:1938
Brandon EJ, Rittenberg DK, Arif AM, Miller JS (1998) Inorg Chem 37:3376
Diskin-Posner Y, Dahal S, Goldberg I (2000) Angew Chem Int Ed Engl 39:1288
Diskin-Posner Y, Goldberg I (1999) Chem Commun 19:1961
Goldberg I (2000) Chem Eur J 6:3863
Collin J-P, Sauvage J-P (1987) J Chem Soc Chem Commun 14:1075
Cox JPL, Jankowski KJ, Kataky R, Parker D, Beeley NRA, Boyce BA, Eaton MAW, Miller K, Millian AT, Harrison A, Walker C (1989) J Chem Soc Chem Commun 12:797
Robert WH, Geoffrey AL, Niel FC (1975) J Chem Soc Perkin Trans 6:1591
Sheldrick GM (1995) SAINT and XPREP, version 5.1. Siemens Industrial Automation Inc., Madison
SADABS (1997) Empirical absorption correction program. University of Göttingen, Gottingen
Sheldrick GM (2008) Acta Crystallogr A Found Crystallogr 64:112
Johnson CK (1976) ORTEPII. Report ORNL-5138. Oak Ridge National Laboratory, Oak Ridge
Spek AL (1999) PLATON a multipurpose crystallographic tool. Utrecht University, Utrecht
Zhou H-B, Liao D-Z, Deng L-X, Yu J-Z, Gao Y-P, Yang X-F, Jiang Z-H, Yan S-P, Cheng P (2006) Struct Chem 17:43
Meghdadi S, Amirnasr M, Mereiter K, Amiri A, Ghodsi V (2010) Inorg Chim Acta 363:1587
Khandar AA, Shaabani B, Belaj F, Bakhtiari A (2006) Polyhedron 25:1893
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feifei, S., Baiwang, S. Synthesis, Crystal Structure and Electrochemical Property of New Rac-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene)bis(azido)cobalt(III) Nitrate Methanol Solvate Complex. J Chem Crystallogr 41, 1790–1793 (2011). https://doi.org/10.1007/s10870-011-9983-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-9983-z