Abstract
The cationic tetranuclear cobalt complex of the geometry Cl-CoL-Cl-CoL-Cl-CoL-Cl-CoL (where L is 6,6′′′-dimethyl-2,2′:6′,2′′:6′′,2′′′-quaterpyridine) has been synthesized and structurally characterized. The structural unit contains one cationic ligand, two Eu(NO3)5 dianions, two solvent acetonitrile molecules and some disordered water molecules. The salt crystallizes in the monoclinic space group C2/c with a = 17.405 (3) Å, b = 28.700 (5) Å and c = 23.350 (5) Å, β = 95.14 (2)º. The cation is C 2 -symmetrical, the central Cl atom lies on the twofold axis. This structure is another example of centro-noncentro ambiguity: in the centrosymmetric model, the terminal Cl in the cation is disordered between the two ends of the chain. The Eu ion is coordinated by five nitro groups, the coordination number is 10–two oxygen atoms of every nitro group are involved in the coordination with the mean Eu–O distance of 2.52(3) Å. The quaterpyridine ligands are folded, the dihedral angle between the terminal ring planes is 29.3 (2) and 32.8 (3)º in the two symmetry independent ligands. In the crystal structure the coulombic attraction between the cations and anions seems to be a driving force; the ionic species make a chessboard-like pattern, the voids in such created structure are filled with the solvent-acetonitrile molecules as well as by some disordered waters.
Graphical Abstract
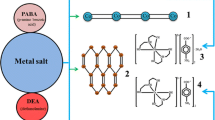
The C 2 -symmetrical cationic complex assumes the unprecedented linear geometry of the form Cl-CoL-Cl-CoL-Cl-CoL-Cl-CoL (where L is tetradentate 6,6′′′-dimethyl-2,2′:6′,2′′:6′′,2′′′-quaterpyridine).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Results and Discussion
The use of transition metal ions capable of bridging multidentate ligands to construct pre-programed, self-assembled small inorganic systems and multi-dimensional infinite supramolecular networks is an area of chemistry which has received ever-increasing attention over recent years. Up to date, many fascinating supramolecular structures have been obtained by using metal ions and ligands containing nitrogen-donor atoms, for instance bipyridine [1], terpyridine [2] or tetrapyridine [3], to name a few.
In the present work, we extend these studies of the metalo-ligand complexes based on tetrapyridine ligand coordinated with d- and f-metal ions, obtained in one-pot reaction. The crystals contain cation with four organic ligands coordinated to the four cobalt ion bridged by chloride ions and two penta-nitrato anionic species with nitrate oxygen atoms coordinated to trivalent europium ion. The formation of this type of complex may be understood considering the large stability of polynitrato counter ions [4]. The high affinity of the europium ions for nitrato anions does not allow heterometallic Co-Eu complexes to be prepared from N4-donor ligand if the latter anions are present in solution [5].
The investigated complex may be of use in several fields of coordination chemistry. In particular, d- and f-polynuclear complexes might be used to fabricate nanomagnetic materials of the potential use in many fields, [6] and due to their electronic properties such as high conductivity as molecular devices in the field of nano-electronics [7, 8].
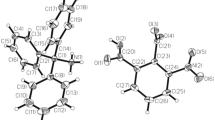
Figures 1 and 2 show the ionic components of the crystal structure. Table 1 lists some relevant geometrical parameters.
Anisotropic ellipsoid representation of the cation together with atom labeling scheme [19]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are depicted as spheres with arbitrary radii. Prime denotes the symmetry code 1−x, −y, 1/2−z
Anisotropic ellipsoid representations of the anion together with atom labeling scheme [19]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are depicted as spheres with arbitrary radii
The cation has the unprecedented geometry of linear four-metal-centered chain. In the Cambridge Crystallographic Database [9] we have not found any complex of similar geometry, besides two anionic metal halides: tris(μ2-iodo)-decakis(iodo)-tetra-mercury(ii) [10] and tris(μ2-fluoro)-dodecafluoro-tetra-antimony [11]. There are also infinite, sometimes branched chains of the ~Me-X-Me-X~ disposition, also with the other ligands connected with metal centers but such a strange disposition is observed—to the best of our knowledge—for the first time. The cation is C 2 -symmetrical, the central Cl atom lies on the twofold symmetry axis. This symmetry constitutes the problem of its own, in fact one of the most important problems of the X-ray crystallography: the centro-noncentro ambiguity (for instance, [12, 13] and references therein). In the present case this ambiguity leads to the choice between one of the two possible models which can be deduced from the same experimental data:
-
centrosymmetric one with the cation in a special position and the terminal chlorine atom with half occupancy (assuming disorder between two oppositely oriented molecules).
-
non-centrosymmetric model in which the space group symmetry is lowered to Cc and the chlorine atom is fully occupied at one of the ends of the molecule.
Of course in principle these two models lead to the same packing; in reality one position of terminal chlorine enforces the same orientation of the neighboring molecule, in order to avoid impossibly short Cl···Cl contacts. So, we have the “homodromic” chains of cations ~Co–Cl~Co–C···Co–Cl~Co–Cl···Co–Cl~Co–Cl~, and the neighboring chains have different orientations (cf. Fig. 3). Due to the space- and time-averaging nature of diffraction experiment both these models lead to the same experimental results. The structure could be solved in both C2/c and Cc space groups, and refinements go more or less properly, but the non-centrosymmetric model leads to large correlations and has no other advantages over the centrosymmetric model; therefore the final refinement was done in the C2/c space group with half-occupied terminal Cl atom. Similar situation, but connected with the hydrogen-bonded chains, was observed for instance in the crystal structure of 1,2-dimethyl-4-nitro-5-morpholino-imidazole hydrate [14].
The neighbouring chains of the cations [20]; for clarity only the “backbone” atoms are shown
The conformation of the backbone of the cation is far from linear, the torsion angles along the chain of Co and Cl atoms are 159.4(4), 143.1 (4), 153.9 (4), 143.1 (4), and 159.4 (4)º. The three Co atoms are six-coordinated (by four nitrogen atoms of L and two Cl), and this coordination is close to square bipyramid (Co–Cl distances are significantly longer than Co–N, cf. Table 1). Only one terminal Co atom (cf. the discussion above) is five-coordinated, with the square pyramidal coordination. The aromatic rings in both symmetry-independent ligands are to a good approximation planar, however the ligands as a whole are far from planarity. The dihedral angles between terminal ring planes, which may serve as a measure of overall twist of these fragments, are 29.3(2) and 32.8 (3)º. More information about the conformation of ligands can be found in Table 2.
In the anion the Eu atom is ten-coordinated (Fig. 2), each nitro group donates two oxygen atom to the coordination sphere. The distances Eu–O with the typical mean value of 2.52 (3) Å show only moderate spread (2.461–2.560 Å), what proves that in fact all ten oxygen atoms take part in the coordination. As expected, the asymmetry in the N–O bond distances is clearly seen: the bonds involving atoms that are engaged in the coordination are significantly longer (mean value 1.285 (20) Å) than those which are involved in intermolecular interactions, if any (1.234 (10) Å).
In the crystal structure these ionic fragments are packed mainly by means of Coulombic interactions; the chessboard-like disposition of cations and anions (Fig. 4) attempts to minimize the repulsion and maximize attraction. The voids in such created structure are filled with ordered solvent (acetonitrile) molecules and some additional electron density which in some instances might be interpreted as disordered water molecules, but there is also some unmodelled residual electron density (cf. Table 2), which is probably caused by totally disordered water and which can be responsible for relatively high R factor of otherwise quite reasonable structure.
Fragment of the crystal packing of 1 as seen along x-direction [20]
Experimental
Preparation
The metal salts were used without further purification as supplied from Aldrich. The ligand L and the complex were prepared as described previously [3]. Solvents were freshly distilled under argon from CaH2. Mass spectra for acetonitrile solutions ~10−4 M were determined using a Waters Micromass ZQ spectrometer. Sample solutions were introduced into the mass spectrometer source with a syringe pump at a flow rate of 40 μL min−1 with a capillary voltage of +3 kV and a desolvation temperature of 300 °C. The source temperature was 120 °C. The cone voltage (Vc) was set to 30 V to allow transmission of ions without fragmentation processes. Scanning was performed from m/z = 200 to 1,000 for 6 s, and 10 scans were summed to obtain the final spectrum. Microanalyses were obtained using a Perkin Elmer 2400 CHN microanalyzer.
Preparation of complex: a mixture of CoCl2·6H2O (6.3 mg, 27 μmol), Eu(NO3)3·5H2O (9.5 mg, 27 μmol) and ligand L (18 mg, 54 μmol) in 2:1 CH3CN-CH2Cl2 (18 mL) was stirred at room temperature for 48 h under argon atmosphere. The volume was reduced under vacuum until crystallization commenced and the mixture was then allowed stand to equilibrate. The pale pink complex was isolated in quantitative yield by evaporation of the solvent (88%). ESI–MS (+): m/z (%) δ = 432 (100) [CoLCl]+, 199 (20) [CoL]2+, ESI–MS (−): m/z (%) = 399 (100) [Eu(NO3)4]−, 231 (20) [Eu(NO3)5]−. Elemental analysis (%) calcd for [Co4(C22H18N4)4Cl4][Eu(NO3)5]2·2CH3CN·3H2O (2807.26): C 39.33; H 3.09; N 13.96; found: C 39.41; H 3.05; N 13.93.
Crystallography
Blue transparent prism-like crystals (0.25, 0.1, 0.1) were used for data collection. Diffraction data were collected at 100 (1) k by the ω-scan technique, on a KUMA KM4CCD diffractometer [15] with graphite-monochromatized MoKα radiation (λ = 0.71073 Å). The temperature was controlled by an Oxford instruments cryosystems cooling device. The crystal quality deteriorated during data collection, which together with the troubles with selecting the specimen of appropriate quality, resulted with the relatively low completeness of the data. Complete room temperature data are of inferior quality. The data were corrected for Lorentz-polarization and absorption effects [15]. Accurate unit-cell parameters were determined by a least-squares fit of 7290 reflections of highest intensity, chosen from the whole experiment. Precision of the diffractometer was also taken into account in order to avoid unphysically small standard uncertainties [16]. The structure was solved by direct methods with SIR92 [17] and refined with the full-matrix least-squares procedure on F2 by SHELXL97 [18]. Scattering factors incorporated in SHELXL97 were used. The function Σw(∣Fo∣2−∣Fc∣2)2 was minimized, with w−1 = [σ2(Fo)2 + (0.06P)2]. Disordered oxygen atoms were refined isotropically, all other non-hydrogen atoms were refined anisotropically, hydrogen atoms were put in the calculated positions difference Fourier map, and refined as a “riding” model with the isotropic displacement parameters set at 1.2 (1.5 for methyl groups) times the U eq value for appropriate non-hydrogen atom. No attempts to find the hydrogen atoms from disordered water molecules were performed. Relevant crystal data are listed in Table 3, together with refinement details.
CCDC-804372 contains supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by e-mailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK.
References
Patroniak V, Stefankiewicz AR, Lehn J-M, Kubicki M (2005) Eur J Inorg Chem 4168
Patroniak V, Baxter PNW, Lehn J-M, Hnatejko Z Kubicki M (2004) Eur J Inorg Chem :2379
Stefankiewicz AR, Wałęsa M, Jankowski P, Ciesielski A, Patroniak V, Kubicki M, Hnatejko Z, Harrowfield JM, Lehn J-M (2008) Eur J Inorg Chem :2910
Radecka-Paryzek W, Patroniak V (1994) Polyhedron 13:2125
Costes J-P, Donnadieu B, Gheorghe R, Novitchi G, Tuchagues J-P, Vendier L (2008) Eur J Inorg Chem :5235
Kartosis G, Evangelisti M, Dalgarno SJ, Brechin EK (2009) Angew Chem Int Ed 48:9928
Wang W-Z, Ismayilov RH, Wang R–R, Huang Y-L, Yeh C-Y, Lee G-H, Peng S-M (2008) Dalton Trans 6:808
Ismayilov RH, Wang W-Z, Lee G-H, Chien C-H, Jiang C-H, Chiu C-L, Yeh C-Y Peng S-M (2009) Eur J Inorg Chem :2110
Allen FH (2002) Acta Crystallogr 58
Dobrzycki L, Wozniak K (2009) J Mol Struct 921:18
Becker IK, Mattes R (1996) Z Anorg Allg Chem 622:105
Schomaker V, Marsh RE (1979) Acta Crystallogr B35:1933
Marsh RE (1999) Acta Crystallogr B35:1933
Kubicki M, Borowiak T, Dutkiewicz G, Sobiak S, Weidlich I (2003) Acta Crystallogr B59:487
Oxford Diffraction (2009) CrysAlis PRO (Version 1.171.33.36d). Oxford Diffraction Ltd, Oxfordshire
Herbstein FH (2000) Acta Crystallogr B56:547
Altomare A, Cascarano G, Giacovazzo C, Gualardi A (1993) J Appl Crystallogr 26:343
Sheldrick GM (2008) Acta Crystallogr A64:112
Siemens (1989) Stereochemical workstation operation manual. Release 3.4. Siemens Analytical X-ray Instruments Inc, Madison
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Crystallogr 41:466
Acknowledgment
This research was carried out as part of the Polish Ministry of Higher Education and Science project (Grant No. NN 204 2716 33).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ciesielski, A., Patroniak, V. & Kubicki, M. Unprecedented Architecture of the Linear (μ-Cl)-Tetranuclear Cobalt Complex with Quaterpyridine Ligand. J Chem Crystallogr 41, 1884–1888 (2011). https://doi.org/10.1007/s10870-011-0195-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0195-3