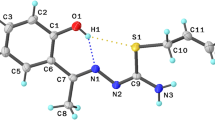
Abstract
The crystal structures of two novel Schiff base hydrazones have been determined by means of the X-ray diffraction. These compounds: N′-[(E)-(2,5-dimethoxyphenyl)methylidene]biphenyl-4-carbohydrazide, C22H20N2O3 (1) and N′-[(E)-(4-fluorophenyl)methylidene]biphenyl-4-carbohydrazide, C20H15FN2O (2), are the first structurally characterized biphenyl derivatives of phenylmethylidene-carbohydrazide. Both compounds crystallize in the monoclinic space groups, 1 in P21/c space group with a = 13.987(2) Å, b = 16.426(3) Å, c = 8.214(2) Å, β = 98.12(2)°, and 2 in C2/c with a = 37.163(5) Å, b = 10.696(2) Å, c = 8.098(2) Å, β = 101.18(2)°. Both molecules have very similar bond lengths and angles pattern, even in the differently substituted phenyl ring. However, the conformations of the molecules differ significantly, the more crowded molecule 1 is much more folded than 2. The dihedral angle between the terminal ring planes is 56.17(6)° in 1 while in 2 it is as small as 2.83(14)°. In both structures relatively short and linear N–H···O hydrogen bonds (created by the best available hydrogen bond donor and acceptor) connect molecules into the chains along the unit cell parameter of ca. 8 Å in length. The next stage of the crystal architecture determination, the secondary interactions, are however quite different: in 1 there are almost solely dispersion van der Waals interactions while in 2 some more specific C–H···F and C–H···π interactions are also involved in the crystal packing.
Graphical Abstract
In both structures relatively short and linear N–H···O hydrogen bonds connect molecules into the chains along the unit cell parameter of ca. 8 Å in length, while the next stages of the crystal architecture, the secondary interactions, are quite different.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff base hydrazones play an important role in inorganic chemistry, as they easily form stable complexes with most transition metal ions. The development of the field of bioinorganic chemistry has increased the interest in Schiff base complexes, since it has been recognized that many of these complexes may serve as models for biologically important species (e.g. [1, 2]). Coordination compounds derived from aroylhydrazones have been reported to act as enzyme inhibitors and are useful due to their pharmacological applications (e.g. [3–5]).
Hydrazones containing an azomethine –NHN=CH– proton are synthesized by heating the appropriate substituted hydrazines/hydrazides with aldehydes and ketones in solvents like ethanol, methanol, tetrahydrofuran, butanol, glacial acetic acid, ethanol–glacial acetic acid. Another synthetic route for the synthesis of hydrazones is the coupling of aryldiazonium salts with active hydrogen compounds [6].
In course of our studies on the different medium and weak interactions that are responsible for the crystal packing we have synthesized and solved the crystal structures of two new Schiff bases, namely N′-[(E)-(2,5-dimethoxyphenyl)methylidene]biphenyl-4-carbohydrazide, C22H20N2O3 (1) and N′-[(E)-(4-fluorophenyl)methylidene]biphenyl-4-carbohydrazide, C20H15FN2O (2), cf. Scheme 1. Although there is a number of crystal structures of benzaldehyde-benzoylhydrazone derivatives (the Version 5.31 of the Cambridge Structural Database [7] updated November 2010 produces 257 hits containing this structural fragment) to the best of our knowledge the compounds described here are the first examples of biphenyl-containing derivatives.
Because the packing of the molecules in crystals results as the compromise between different factors including e.g. intermolecular interactions, studying of the packing regularities and differences in the crystals built of similar molecules might be useful in the area of supramolecular chemistry (e.g. [8, 9] and references therein). In both 1 and 2 one can find one good hydrogen bond donor (NH) and one C=O group that might act as hydrogen bond acceptor. Therefore it could be anticipated that the main packing motif is a chain of hydrogen bond molecules, probably related by a screw axis or a glide plane. However the packing of the chains has to be determined by other interactions and requirements, which in this case would be among such factors as close packing, van der Waals interactions, weak hydrogen bonds (C–H···O, F, N or π), π–π stacking etc. Studies on such closely related but different systems might be useful in gaining the better understanding of the factors that influence the crystal packing.
Experimental
Synthesis
Synthesis of N′-[(E)-(2,5-dimethoxyphenyl)methylidene]biphenyl-4-carbohydrazide (1)
Biphenyl-4-carbohydrazide (0.01 mol, 2.12 g) and 2,5-dimethoxy benzaldehyde (1.66 g, 0.01 mol) were dissolved in ethanol (30 mL) and added two drops of Conc. HCl. Refluxed the mixture for about 3 h. On cooling, the solid separated was filtered, washed with water and dried. Good quality crystals were grown from DMF solution by slow evaporation (m.p.: 495 K). Composition: Found (Calculated): C: 38.39 (38.47); H: 3.17 (3.23); N: 22.35% (22.43%).
Synthesis of N′-[(E)-(4-fluorophenyl)methylidene]biphenyl-4-carbohydrazide (2)
Biphenyl-4-carbohydrazide (0.01 mol, 2.12 g) and 4-fluoro benzaldehyde (1.24 g, 0.01 mol) were dissolved in ethanol (30 mL) and added two drops of Conc. HCl. Refluxed the mixture for about 3 h. On cooling, the solid separated was filtered, washed with water and dried. Good quality crystals were grown from DMF solution by slow evaporation (m.p.: 519 K). Composition: Found (Calculated): C: 75.39(75.46); H: 4.68 (4.75); N: 8.74% (8.80%).
X-ray Structure Determination
Diffraction data were collected at room temperature by the ω-scan technique, on an Oxford Diffraction SuperNova four-circle diffractometer equipped with Atlas CCD-detector [10] using mirror-monochromatized CuKα radiation from high-flux micro-focus source (λ = 1.54178 Å). The data were corrected for Lorentz-polarization as well as for absorption effects [10]. Accurate unit-cell parameters were determined by a least-squares fit of 5001 (1), and 7561 (2) reflections of highest intensity, chosen from the whole experiment. The structures were solved with SIR92 [11] and refined with the full-matrix least-squares procedure on F 2 by SHELXL97 [12]. Scattering factors incorporated in SHELXL97 were used. The function Σw(∣F o∣2 − ∣F c∣2)2 was minimized, with w −1 = [σ2(F o)2 + (AP)2 + BP], where P = [Max (F 2o , 0) + 2F 2c /3]. The final values of A and B are listed in Table 1 together with relevant crystal data and refinement details. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms from the methyl groups in 1 were placed geometrically, in idealized positions (C–H distances of 0.96 Å), and refined as rigid groups with their U iso’s as 1.5 times U eq of the appropriate carrier atom. All other hydrogen atoms were found in the difference Fourier maps and isotropically refined.
Crystallographic data (excluding structure factors) for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, Nos. CCDC-813832 (1), and CCDC-813833 (2). Copies of this information may be obtained free of charge from: The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK. Fax: +44(1223)336-033, e-mail:deposit@ccdc.cam.ac.uk, or www: www.ccdc.cam.ac.uk.
Results
Figures 1 and 2 show the perspective views of the molecules 1 and 2, respectively. Table 2 compares the relevant geometric parameters of both molecules. The bond lengths and angles in both compounds are very similar; a majority of them differ by less than 3σ, and even the results of the normal probability plot test [13, 14] confirm that the differences between the molecules are mainly of statistic nature.
Anisotropic ellipsoid representation of the compound 1 together with atom labeling scheme [11]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are shown as spheres of arbitrary radii
Anisotropic ellipsoid representation of the compound 2 together with atom labeling scheme [11]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are shown as spheres of arbitrary radii
The correlation coefficient R 2 between the set of experimental differences between the geometrical parameters and the theoretical values for pure statistical distribution is 0.967 for the bond lengths (excluding C14–C141 and C14–F14 bonds) and 0.984—for angles. Of course the different substituents in ring A cause the differences in the intraannular bond angles patterns—as observed for instance by Domenicano and Murray-Rust [15, 16]—but it seems that in this case, due to the various positions of these substituents, the differences mainly cancel out.
The overall conformations of 1 and 2 differ, and it can be related to the presence or absence of the substituent in the ortho position of the ring A, even though it seems that there is enough space for methoxy group to accommodate and the CSD data do not show the clear conformational preferences. The overall shape of these molecules can be described by the dihedral angles between three planar fragments (cf. Fig. 1 for the nomenclature; Table 2 for appropriate values). In 1 the central C=N–N–C=O chain is relatively far from the planarity, the atoms N8, N9, C10, O11 and C12 are coplanar within 0.018 Å while N7 deviates from that mean plane by as much as 0.488(2) Å. This mean plane makes the dihedral angle of 50.68(6)° with the plane of ring A and 39.92(5)° with that of ring B. In 2 the whole C=N–N–C=O chain is planar within 0.019(1) Å, and it makes similar to previously given dihedral angle with B, 33.32(15)°, while the twist with respect to the ring A is significantly smaller, 12.89(15)°. The biphenyl fragments in both molecules are twisted but also in this case the twist angle is greater in 1, the dihedral angle between mean planes of phenyl rings is 33.48(5)° in 1 and 23.14(10)° in 2. The terminal rings A and C are significantly twisted in 1 (56.17(6)°) while they are almost coplanar in 2 (2.83(14)°). The comparison of both molecules, fitted onto their rings A, is shown in Fig. 3. The geometries of both 1 and 2 are well within the typical ranges.
The comparison of the molecules of 1 (dashed) and 2 (solid); the rings A were fitted onto one another [11]
These quite closely related molecules turned out to show to some extent different organisation in the crystal structure. As expected, in both cases the N–H···O hydrogen bonds, relatively linear, connect molecules into the infinite chains of molecules connected by the c-glide plane. In both cases therefore the chains extend along [001] direction (cf. Fig. 4a, b; Table 3), and in both structures—despite different space groups—the unit cell parameters c have similar values (8.214 Å in 1, 8.098 Å in 2).
The hydrogen bonded chains of molecules 1 (a) and 2 (b) as seen approximately along y direction. Hydrogen bonds are shown as dashed lines [11]
The next levels of the structure organizations are however different, which can be caused by the differences in the molecular conformations. In 1 there are virtually no specific interactions which might play a role in the designing of the crystal structure. Therefore only close packing requirements and van der Waals forces are involved in the crystal structure. Contrary, in 2 there is some weak but definitely directional C–H···F interactions, and two—probably also of some importance—C–H···π contacts with aromatic rings playing role of very weak acceptor of a hydrogen-bond-like interaction (cf. Table 3). These subtle differences lead to quite dissimilar mode of packing of the hydrogen-bonded chains (Fig. 5).
The crystal packing as seen along the direction of hydrogen bonded chains in 1 (a) and 2 (b) [11]
References
Jeeworth T, Wah HLK, Bhowon MG, Ghoorhoo D, Babooram K (2000) Synth React Inorg Met-Org Chem 30:1023
Dharmaraj N, Viswanalhamurthi P, Natarajan K (2001) Transit Met Chem 26:105
Savanini L, Chiasserini L, Gaeta A, Pellerano C (2002) Biorg Med Chem 10:2193
Ferrari MB, Capacchi S, Pelosi G, Reffo G, Tarasconi P, Alberlini R, Pinelli S, Lunghi P (1999) Inorg Chim Acta 286:134
Dharmaraj N, Viswanalhamurthi P, Natarajan K (2001) Transit Met Chem 26:105
Rollas S, Kucukguzel SG (2007) Molecules 12:1910
Allen FH (2002) Acta Crystallogr B58:380
Brock CP, Dunitz JD (1994) Chem Mater 6:1118
Kubicki M (2005) J Mol Struct 743:209
Agilent Technologies (2010) CrysAlis PRO (version 1.171.35.4). Agilent Technologies Ltd, Yarnton
Altomare A, Cascarano G, Giacovazzo C, Gualardi A (1993) J Appl Crystallogr 26:343
Sheldrick GM (2008) Acta Crystallogr A64:112
Abrahams SC, Keve ET (1971) Acta Crystallogr A27:157
(1974) International tables for X-ray crystallography, vol IV. Kluwer, Dordrecht, p 293
Domenicano A, Murray-Rust P (1979) Tetrahedron Lett 24:2283
Domenicano A (1988) In: Hargittai I, Hargittai M (eds) Stereochemical applications of gas-phase electron diffraction. Part B: Structural information for selected classes of compounds. VCH, Weinheim, p 281
Acknowledgment
BN thanks UGC, New Delhi, Government of India for the purchase of chemicals through SAP-DRS-Phase 1 programme.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dutkiewicz, G., Narayana, B., Samshuddin, S. et al. Synthesis and Crystal Structures of Two New Schiff Base Hydrazones Derived from Biphenyl-4-Carbohydrazide. J Chem Crystallogr 41, 1442–1446 (2011). https://doi.org/10.1007/s10870-011-0118-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0118-3