Abstract
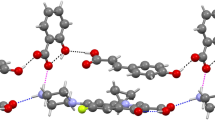
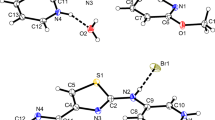
In the title compound, [C14H19N4O3 +, C9H5 O2 −, H2O, O0.52] the asymmetric unit contains a protonated trimethoprim cation and a cinnamate anion and two water molecules. The crystal structure was determined by single crystal X-ray diffraction. This compound crystallized in the triclinic system; space group P−1 with the unit cell parameters a = 10.010(2) Å, b = 10.339(3) Å, c = 13.486(8) Å, α = 105.32(3)°, β = 109.88(3)°, γ = 100.89(3)°, V = 1204.6(10) Å3, Z = 2. The cinnamate group is disordered. The trimethoprim (TMP) molecule is protonated at one of the pyrimidine nitrogen atoms. The carboxylate group of the cinnamate anion interacts with the protonated pyrimidine atom N1 and the 2-amino group via a pair of N–H···O hydrogen bonds, generating the R 22 (8) ring motif. The inversion related TMP cations are paired via N–H···N hydrogen bonds. In addition to the base pairing, the O1W atom bridges the 2-amino and 4-amino groups on either side of the paired bases, resulting in a self complementary DADA array. Two inversion related TMP cations and water molecules (O1W) are linked via N–H···O and O–H···O hydrogen bonds, forming a 22 membered ring with graph-set R 44 (22).
Graphical Abstract
The protonated trimethoprim cation interacts with the cinnamate anion through a pair of N-H…O hydrogen bonds, generating the R 22 (8) ring motif.

.



Similar content being viewed by others
References
Lehn J-M (1995) Supramolecular chemistry. VCH, Weinheim
Desiraju GR (1989) Crystal engineering: the design of organic solids. Elsevier, Amsterdam
Moulton B, Zaworotko MJ (2001) Chem Rev 101:1629–1658
Desiraju GR (2001) Nature 412:397–400
Desiraju GR (2001) Curr Sci 81:1038–1042
Desiraju GRJ (2003) Mol Struct 656:5–15
Etter MC, Adsmond DA (1990) J Chem Soc Chem Commun 589–591
Goswami S, Mahapatra AK, Nigam GD, Chinnakali K, Fun H-K, Razak IA (1999) Acta Crystallogr Sect C 55:583–585
Pedireddi VR, Chatterjee S, Ranganathan A, Rao CNR (1998) Tetrahedron 54:9457–9474
Koetzle TF, Williams GJBJ (1976) Am Chem Soc 98:2074–2078
Bettinetti GP, Giordano F, La Manna A, Giuseppetti G, Tadini C (1985) Acta Crystallogr Sect C 41:1249–1253
Bryan RF, Haltiwanger RC, Woode MK (1987) Acta Crystallogr Sect C 43:2412–2415
Schwalbe CH, Cody V (2006) Crystallogr Rev 4:267–300
Murugesan S, Muthiah PT (1997) Acta Crystallogr Sect C 53:763–764
Muthiah PT, Umadevi B, Stanley N, Shui X, Eggleston DS (2001) Acta Crystallogr Sect E 57:o1179–o1182
Stanley N, Muthiah PT, Geib SJ, Luger P, Weber M, Messerschmidt M (2005) Tetrahedron 61:7201–7210
Hemamalini M, Muthiah PT, Lynch DE (2005) Acta Crystallogr Sect E 61:o4107–o4109
Subashini A, Samuel E, Muthiah PT, Bocelli G, Cantoni A (2007) Acta Crystallogr Sect E 63:o4049–o4049
Prabakaran P, Robert JJ, Muthiah PT, Bocelli G, Righi L (2001) Acta Crystallogr Sect C 57:459–461
Robert JJ, Raj SB, Muthiah PT (2001) Acta Crystallogr Sect E 57:o1206–o1208
Francis S, Muthiah PT, Bocelli G, Righi L (2002) Acta Crystallogr Sect E 58:o717–o719
Stanley N, Sethuraman V, Muthiah PT, Luger P, Weber M (2002) Cryst Growth Des 2:631–635
Raj SB, Muthiah PT, Rychlewska U, Warzajtis B (2003) Cryst Eng Comm 5:48–53
Sethuraman V, Stanley N, Muthiah PT, Sheldrick WS, Winter M, Luger P, Weber M (2003) Cryst Growth Des 3:823–828
Siemens XSCANS (1994) Version 2.1 Siemens Analytical X-ray instrument Inc, Madison
Sheldrick GM (1997) SHELXS-97 and SHELXL-97. University of Gottingen, Gottingen
Spek AL (2003) J. Appl Cryst 36:7–13
Hitchings GH, Kuyper LF, Baccananari DP (1988) In: Sandler M, Smith HJ (eds) Design of enzyme inhibitors as drugs. Oxford University Press, New York
Giuseppetti G, Tadini C, Bettinetti GP, Giordano F, La Manna A (1988) Acta Crystallogr Sect C 44:856–860
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34:1555–1573
Kuyper LF (1990) In: Bugg CE, Ealick SE (eds) Crystallographic and modeling methods in molecular design. Springer Verlag, New York, pp 56–79
Allen FH, Raithby PR, Shields GP, Taylor R (1998) Chem Commun 9:1043–1044
Panneerselvam P, Stanley N, Muthiah PT (2002) Acta Crystallogr Sect E 58:o180–o182
Acknowledgment
AS thanks Bharathidasan University, Tiruchirappalli, Tamil Nadu, India for the Award of a Research Studentship [Ref: CCCD/Ph.D-2/15504/2004].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subashini, A., Muthiah, P.T., Bocelli, G. et al. Hydrogen Bonding Patterns in Trimethoprimium Cinnamate 1.52 Hydrate. J Chem Crystallogr 41, 976–979 (2011). https://doi.org/10.1007/s10870-011-0028-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0028-4