Abstract
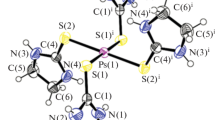
We wish to report the crystal structure for a solid-state solution, {[Pt(9S3)Cl2]2[Pt(9S3)2]}Cl2·4H2O (1), which contains two different complexes of Pt(II) with the trithiacrown 9S3 (1,4,7-trithiacyclononane). One complex [Pt(9S3)Cl2] is neutral and contains a Pt(II) center with a single 9S3 ligand and two coordinated chloro ligands. The second Pt(II) complex is a dication and contains two coordinated 9S3 ligands and two non-coordinating chloride anions. There are two crystallographically equivalent [Pt(9S3)Cl2] complexes present for every single [Pt(9S3)2]2+ cation. Four water solvent molecules are also present. In the neutral complex ([Pt(9S3)Cl2], the Pt(II) center is surrounded by a cis-[S2Cl2 + S1] ligand environment formed by the two chloro ligands and two of the three sulfur atoms from the 9S3 ligand. These two sulfurs are positioned 2.225(2) and 2.242(2) Å from the Pt(II), but the third sulfur shows a long distance interaction at 3.311(2) Å to form an elongated square pyramidal structure. This axial Pt–S distance is the longest observed in 57 crystal structures of Pt(II) 9S3 complexes. The cation [Pt(9S3)2]2+ displays two centrosymmetrically coordinated 9S3 ligands forming a [S4 + S2] environment with an elongated octahedral shape. In the dication, the two equatorial Pt–S distances are 2.297(2) and 2.306(2) Å with the axial sulfur at 3.065(2) Å. The most interesting intermolecular aspects of the structure are hydrogen bonding interactions between two of the water molecules and two chloride counter-ions, resulting in a nearly square O2Cl2 ring. This ring shares an edge with a six-membered ring formed by four waters and two chlorides which are hydrogen bonded. The hydrogen bonding interactions, which result from the presence of water in the crystal, appear to be an important component in stabilizing the lattice for the unusual solid-state solution structure. Crystal Data for (1): P2 1 /n, a = 7.8327(10) Å, b = 25.152(4) Å, c = 12.382(2) Å, V = 2314.3(6) Å3, Z = 2. We also report an improved synthetic procedure for the preparation of two thiacrown complexes [Pt(9S3)Cl2] and [Pt(10S3)Cl2], which are commonly used as precursors for other heteroleptic thioether complexes. The new syntheses proceed at room temperature without the need for long reflux times and produce large crystals which are easily isolable without filtration. The simplicity of these new preparations results in improved yields over previously employed methods.
Graphical Abstract
The title complex crystallizes to form a solid-state solution of two different Pt(II) thiacrown complexes, [Pt(9S3)Cl2] and [Pt(9S3)]2+.



Similar content being viewed by others
References
Blake AJ, Schröder M (1990) In: Sykes AG (ed) Advances in inorganic chemistry, vol 35. Academic Press, Inc., New York, p 2
Cooper S, Rawle SC (1990) Struct bonding (Berlin) 72:1
Setzer WN, Cacioppo EL, Guo Q, Grant GJ, Kim DD, Hubbard JL, VanDerveer DG (1990) Inorg Chem 29:2672
Blake AJ, Holder AJ, Hyde TI, Roberts YV, Lavery AJ, Schröder M (1987) J Organomet Chem 323:261
Blake AJ, Holder AJ, Hyde TI, Schröder M (1987) J Chem Soc Chem Commun 987
Grant GJ, Spangler NS, Setzer WN, VanDerveer DG (1996) Inorg Chim Acta 246:41
Blake AJ, Holder AJ, Roberts YV, Schröder M (1990) Acta Crystallogr C 44:360
Bennett MA, Felixberger JK, Willis AC (1993) Gazz Chim Ital 123:405
Grant GJ, Brandow CG, Galas DF, Davis JP, Pennington WT, Zubkowski JD, Valente EJ (2001) Polyhedron 20:3333
Lee G-H (1998) Acta Crystallogr C54:906
Blake AJ, Roberts YV, Schröder M (1996) J Chem Soc Dalton Trans 1885
Grant GJ, Galas DF, Poullaos IM, Carter SM, VanDerveer DG (2002) J Chem Soc Dalton Trans 15:2973
Grant GJ, Poullaos IM, Galas DF, VanDerveer DG, Zubkowski JD, Valente EJ (2001) Inorg Chem 40:564
Grant GJ, Pool JP, VanDerveer DG (2003) J Chem Soc Dalton Trans 3981
Bennett MA, Canty AJ, Felixberger JK, Rendina LM, Sunderland C, Willis AC (1993) Inorg Chem 32:1951
Nikol H, Bürgi H-B, Hardcastle KI, Gray HB (1995) Inorg Chem 34:6319
Grant GJ, Patel KN, Helm ML, Mehne LF, Klinger DW, VanDerveer DG (2004) Polyhedron 23:1361
Janzen DE, VanDerveer DG, Mehne LF, da Dilva Fihlo AA, Bredas J-L, Grant GJ (2008) Dalton Trans 1872
Green TW, Lieberman R, Mitchell N, Krause Bauer JA, Connick WB (2005) Inorg Chem 44:1955
Janzen DE, Patel KN, VanDerveer DG, Grant GJ (2006) Chem Commun 3540
Bedford RB, Betham M, Butts CP, Coles SJ, Hursthouse MB, Scully PN, Tucker JHR, Wilkie J, Willener Y (2008) Chem Commun 2429
Janzen DE, Mehne LF, VanDerveer DG, Grant GJ (2005) Inorg Chem 44:8182
Shin RYC, Goh CL, Goh LY, Webster RD, Li Y (2009) Eur J Inorg Chem 2282
Chatterjee S, Krause JA, Connick WB, Genre C, Rodrigue-Withcel A, Reber C (2010) Inorg Chem 49:2808
Pierce E, Lanthier E, Genre C, Chumakov Y, Luneau D, Reber C (2010) Inorg Chem 49:4901
Wieghardt K, Küppers H-J, Raabe E, Krüger C (1996) Angew Chem Int Ed Engl 25:1101
APEX2 Version 2.2, SAINT Version 7.34a (2007) Bruker AXS Inc
SADABS Version (2007/2), XPREP Version (2005/2) (Sheldrick, Bruker AXS Inc)
Sheldrick GM (2008) Acta Cryst A64:112–122 (XS Version 2008/1, XL Version 2008/1)
Grant GJ, Naik RD, Janzen DE, Benefield DA, VanDerveer DG (2010) Supramol Chem 22:109
Cambridge Structural Database v 5.31, November, 2009 + Feb. 2010 and May 2010 updates; Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ. U.K
Blake AJ, Gould RO, Holder TI, Lavery AJ, Odulate MO, Schröder M (1987) J Chem Soc Chem Commun 118
Grant GJ, Chen W, Goforth AM, Baucom CL, Patel K, Repovic P, VanDerveer DG, Pennington WT (2005) Eur J Inorg Chem 479
Acknowledgments
This research was generously supported by grants from the Petroleum Research Fund, administered by the American Chemical Society, the Grote Chemistry Fund at the University of Tennessee at Chattanooga, and by the National Science Foundation, Research at Undergraduate Institutions Program. The assistance with aspects of the synthetic work by Mr. Keith Sanders and Ms. Melissa Brown from the Girls Preparatory School in Chattanooga is appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grant, G.J., Benefield, D.A., Naik, R.D. et al. The Crystal Structure of {[Pt(9S3)Cl2]2[Pt(9S3)2]}Cl2·4H2O: An Improved Synthesis of [Pt(9S3)Cl2]. J Chem Crystallogr 41, 847–853 (2011). https://doi.org/10.1007/s10870-011-0011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0011-0