Abstract
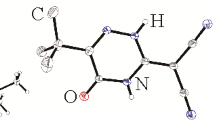
The structures of two polymorphs of the anhydrous cocrystal adduct of bis(quinolinium-2-carboxylate) DL-malic acid, one triclinic the other monoclinic and disordered, have been determined at 200 K. Crystals of the triclinic polymorph 1 have space group P-1, with Z = 1 in a cell with dimensions a = 4.4854(4), b = 9.8914(7), c = 12.4670(8) Å, α = 79.671(5), β = 83.094(6), γ = 88.745(6)°. Crystals of the monoclinic polymorph 2 have space group P21/c, with Z = 2 in a cell with dimensions a = 13.3640(4), b = 4.4237(12), c = 18.4182(5) Å, β = 100.782(3)°. Both structures comprise centrosymmetric cyclic hydrogen-bonded quinolinic acid zwitterion dimers [graph set R 22 (10)] and 50% disordered malic acid molecules which lie across crystallographic inversion centres. However, the oxygen atoms of the malic acid carboxylic groups in 2 are 50% rotationally disordered whereas in 1 these are ordered. There are similar primary malic acid carboxyl O–H···Oquinaldic acid hydrogen-bonding chain interactions in each polymorph, extended into two-dimensional structures but in 1 this involves centrosymmetric cyclic head-to-head malic acid hydroxyl-carboxyl O–H···O interactions [graph set R 22 (10)] whereas in 2 the links are through single hydroxy-carboxyl hydrogen bonds.
Graphical Abstract
The structure determinations of two crystal polymorphs of the 2:1 adduct of quinolinium-2-carboxylate with DL-malic acid has shown one to be triclinic and ordered while in the second monoclinic form the carboxylic acid groups of the malic acid moiety are disordered.








Similar content being viewed by others
References
O’Neil MJ (ed) (2001) The Merck index, 13th edn. Merck & Co. Inc, Whitehouse Station, NJ, USA, p 1440
Dobrzyńska D, Jerzykiewicz LB (2004) J Chem Crystallogr 34:51
Etter MC, MacDonald JC, Bernstein J (1990) Acta Crystallogr B46:256
Smith G, Wermuth UD, White JM (2006) Acta Crystallogr C62:o694
Smith G, Wermuth UD, White JM (2004) Acta Crystallogr C60:o575
Smith G, Wermuth UD, White JM (2008) Acta Crystallogr E64:o132
Smith G, Wermuth UD, White JM (2008) Acta Crystallogr C64:o180
Smith G, Wermuth UD, White JM (2007) Aust J Chem 60:264
Raisanen MT, Klinga M, Leskela M, Repo T (2007) Acta Crystallogr E63:o1926
Farrell DMM, Ferguson G, Lough AJ, Glidewell C (2002) Acta Crystallogr B58:530
CrysAlisPro (2010) (version 1.171.33.55). Oxford Diffraction Ltd., Yarnton, England
Sheldrick GM (1996) SADABS: Absorption correction program for area detectors. University of Göttingen, Germany
Sheldrick GM (2008) SHELXS97 and SHELXL97: Programs for single crystal structure solution and refinement. Acta Crystallogr A64:112
Farrugia LJ (1999) WinGX, A suite for small-molecule single-crystal crystallography. J Appl Crystallogr 32:837
Spek AL (2009) PLATON: a multipurpose crystallographic tool. Acta Crystallogr D65:48
Acknowledgments
The authors acknowledge financial support from the Australian Research Council, the Faculty of Science and Technology (Queensland University of Technology) and the School of Biomolecular and Physical Sciences, Griffith University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, G., Wermuth, U.D. Order and Disorder in the Structures of Two Crystal Polymorphs of the Adduct Bis(Quinolinium-2-Carboxylate) DL-Malic Acid. J Chem Crystallogr 41, 241–246 (2011). https://doi.org/10.1007/s10870-010-9871-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9871-y