Abstract
The single-crystal X-ray structures and in vivo activities of three aryl acetylenic inhibitors of cytochromes P450 1A1, 1A2, 2A6, and 2B1 have been determined and are reported herein. These are 1-ethynylpyrene, 1-propynylpyrene, and 4-propynylpyrene. To investigate electronic influences on the mechanism of enzyme inhibition, the experimental electron density distribution of 1-ethynylpyrene has been determined using low-temperature X-ray diffraction measurements, and the resulting net atomic charges compared with various theoretical calculations. A total of 82,390 reflections were measured with Mo Kα radiation to a (sinθ/λ)max = 0.985 Å−1. Averaging symmetry equivalent reflections yielded 8,889 unique reflections. A least squares refinement procedure was used in which multipole parameters were added to describe the distortions of the atomic electron distributions from spherical symmetry. A map of the model electron density distribution of 1-ethynylpyrene was obtained. Net atomic charges calculated from refined monopole population parameters yielded charges that showed that the terminal acetylenic carbon atom (C18) is more negative than the internal carbon (C17). Net atomic charges calculated by ab initio, density functional theory, and semi-empirical methods are consistent with this trend suggesting that the terminal acetylenic carbon atom is more likely to be the site of oxidation. This is consistent with the inhibition mechanism pathway that results in the formation of a reactive ketene intermediate. This is also consistent with assay results that determined that 1-ethynylpyrene acts as a mechanism-based inhibitor of P450s 1A1 and 1A2 and as a reversible inhibitor of P450 2B1. Crystallographic data: 1-ethynylpyrene, C18H10, P21/c, a = 14.571(2) Å, b = 3.9094(5) Å, c = 20.242(3) Å, β = 105.042(2)°, V = 1,113.5(2) Å3; 1-propynylpyrene, C19H12, P21/n, a = 8.970(2) Å, b = 10.136(1) Å, c = 14.080(3) Å, β = 99.77(2)°, V = 1,261.5(4) Å3; 4-propynylpyrene, C19H12, Pbca, a = 9.904(1) Å, b = 13.174(2) Å, c = 19.401(1) Å, V = 2,531.4(5) Å3.
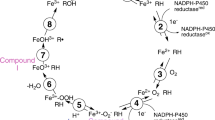
Graphical Abstract
The experimental electron density distribution of 1-ethynylpyrene as well as the single-crystal X-ray structures and in vivo inhibition activities of 1-ethynylpyrene, 1-propynylpyrene, and 4-propynylpyrene have been determined and are reported herein.





Similar content being viewed by others
Notes
Conventional reliability indices: \( R = \sum \left\| {F_{ 0} } \right.| - |\left. {F_{\text{c}} } \right\|/\sum |F_{\text{o}} |\,{\text{and}}\,R_{w} = \left( {\sum \left( {F_{\text{o}} | - |F_{\text{c}} |} \right)^{2} /\sum w|F_{ 0} |^{2} } \right)^{1/2} \), where F o and F c are observed and calculated structure factors, w = 1/σ(F)2, and where σ(F) is the estimated standard deviation in F o.
The supplementary crystallographic data for (a) 1-ethynylpyrene (1-EP) (CCDC 731480), (b) 1-propynylpyrene (1-PP) (CCDC 731481), and (c) 4-propynylpyrene (4-PP) (CCDC 731482), have been deposited. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
SMART Software, Bruker-AXS, 6300 Enterprise Dr., Madison, WI 53719-1173, (1994); SAINT Software, Bruker-AXS, 6300 Enterprise Dr., Madison, WI 53719-1173, (1995).
References
Ortiz de Montellano PR (ed) (1995) Cytochrome P450 structure, mechanism, and biochemistry, 2nd edn. Plenum Press, New York, pp 1–652
Estabrook RW (1996) The remarkable P450s: a historical overview of these versatile hemeprotein catalysts. FASEB J 10:202–204
Masters BSS (1996) Introduction: cytochrome P450. FASEB J 10(2):205
Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR (1995) Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem 270:11595–11602
Schenkman JB, Greim H (eds) (1993) Cytochrome P450 handbook of experimental pharmacology, vol 105. Springer, Berlin, pp 1–739
Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J (1995) Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure 3(1):41–62
Alexander DL, Jefcoate CR (1995) Proc Am Assoc Cancer Res 36, 152 abstract 905
Foroozesh M, Primrose G, Guo Z, Bell LC, Alworth WL, Guengerich FP (1997) Aryl acetylenes as mechanism-based inhibitors of cytochrome P450-dependent monooxygenase enzymes. Chem Res Toxicol 10(1):91–102
Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WA, Guengerich FP (1998) Selectivity of polycyclic inhibitors for human cytochrome P450 1A1, 1A2, and 1B1. Chem Res Toxicol 11(9):1048–1056
Strobel SM, Szklarz GD, He YQ, Foroozesh M, Alworth WL, Roberts ES, Hollenberg PF, Halpert JR (1999) Identification of selective mechanism-based inactivators of cytochrome P450 2B4 and 2B5, and determination of the molecular basis for differential susceptibility. J Pharmacol Exp Ther 290:445–451
Komives EA, Ortiz de Montellano PR (1987) Mechanism of oxidation of π bonds by cytochrome P-450: electronic requirements of the transition state in the turnover of phenylacetylenes. J Biol Chem 262:9793–9802
CaJacob CA, Chan WK, Shephard E, Ortiz de Montellano PR (1988) The catalytic site of rat hepatic lauric acid ω-hydroxylase: protein versus prosthetic heme alkylation in the ω-hydroxylation of acetylenic fatty acids. J Biol Chem 263:18640–18649
Mackay S, Gilmore CJ, Edwards C, Stewart N, Shankland K (1999) MaXus computer program for the solution and refinement of crystal structures. Bruker Nonius, The Netherlands, MacScience, Japan and The University of Glasgow
(1974) International tables for X-ray crystallography, vol IV, pp 71–151, Kynoch Press, Birmingham, England
Sheldrick GM (1997) SHELXL9. Program for the refinement of crystal structures. University of Gottingen, Germany
Koritsanszky T, Howard ST, Su Z, Mallinson PR, Richter T, Hansen NK (1995) XD: a computer program package for multipole refinement and analysis of electron densities from diffraction data. Free University of Berlin, Germany
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2002) Gaussian 98, revision A.11.4. Gaussian Inc., Pittsburgh
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17(5–6):490–519
Stewart JJP (1994) MOPAC 6.0. Fujitsu Limited, Tokyo, Japan
(2004) SYBYL molecular modeling software, versions 7.2. Tripos Associates Inc., St. Louis, Missouri 63144
Burke MD, Thompson S, Weaver RJ, Wolf CR, Mayer RT (1994) Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem Pharmacol 48:923–936
Buters JT, Schiller CD, Chou RC (1993) A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog, and man. Biochem Pharmacol 46:1577–1584
Deluca JG, Dysaft GR, Rasnick D, Bradley MO (1989) A direct, highly sensitive assay for cytochrome P-450 catalyzed O-deethylation using a novel coumarin analog. Biochem Pharmacol 37:1731–1739
Hopkins NE, Foroozesh MK, Alworth WL (1992) Suicide inhibitors of cytochrome P450 1A1 and P450 2B1. Biochem Pharmacol 44:787–796
Acknowledgments
We would like to acknowledge the National Institutes of Health MBRS SCORE (Grant No. 1S06GM08008 and 1SC1GM084722) and RISE (Grant No. 2R25GM060926) Programs for support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, N., Lightsey, D., Liu, J. et al. Ethynyl and Propynylpyrene Inhibitors of Cytochrome P450. J Chem Crystallogr 40, 343–352 (2010). https://doi.org/10.1007/s10870-009-9659-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9659-0