Abstract
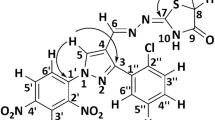
The structures of eight related thio(semi)carbazones are described. These are syn,1E-2-acetylpyrazine-3-thiosemicarbazone (1), syn,1E,4Z-2-acetylpyrazine-4-ethyl-3-thiosemicarbazone, (2) and syn,1Z-2-acetylpyrazine-4,4-dimethyl-3-thiosemicarbazone (3), syn,1E,4Z-2-acetylthiazole-4-phenyl-3-thiosemicarbazone (4), syn,1Z-phenyl-4,4-dimethyl-3-thiosemicarbazone (5), syn,1E,4Z-phenyl-4-methyl-3-thiosemicarbazone (6), syn,1E,4Z-phenyl-4-ethyl-3-thiosemicarbazone (7), syn,1E,4Z-2-acetophenone-5-(N-aminothionyl)-3-thiocarbazone (8). Crystal data: for 1: triclinic, P-1, a = 5.4053(10) Å, b = 7.435(3) Å, c = 11.772(4) Å, α = 81.70(3)°, β = 82.59(2)°, γ = 77.38(2)°, and Z = 2: for 2: triclinic, P-1, a = 7.322(3) Å, b = 7.8239(16) Å, c = 9.783(4) Å, α = 87.73(2)°, β = 79.46(3)°, γ = 80.41(2)°, and Z = 2; for 3: orthorhombic, Pnma, a = 13.5210(15) Å, b = 6.6914(5) Å, c = 11.7214(10) Å, and Z = 4; for 4: triclinic, P-1, a = 5.7058(7) Å, b = 9.8776(15) Å, c = 11.869(2) Å, α = 76.389(12)°, β = 86.364(13)°, γ = 88.322(12)°, and Z = 2; for 5: triclinic, P-1, a = 7.5362(3) Å, b = 8.6331(4) Å, c = 9.8753(4) Å, α = 91.401(4)°, β = 102.532(4)°, γ = 110.540(4)°, and Z = 2; for 6: monoclinic, P2(1)/c, a = 10.7178(4) Å, b = 5.5866(2) Å, c = 18.7647(9) Å, β = 104.392(5)°, and Z = 4; for 7: monoclinic, P2(1)/c, a = 8.9557(3) Å, b = 9.7418(4) Å, c = 13.6864(5) Å, β = 94.044(4)°, and Z = 4; for 8: triclinic, P-1, a = 9.8091(5) Å, b = 10.6173(4) Å, c = 16.4691(7) Å, α = 75.540(4)°, β = 80.305(4)°, γ = 65.192(4)°, and Z = 4. Structures 1, 2, 4, 5, 7, and 8 form intramolecular N4–H···N1 hydrogen-bonds. Structures 3 and 5 exist in the tautomeric form in which N1 is protonated.
Graphical Abstract
Structures of thio(semi)carbazones were found to fall into two categories: those with intramolecular N4-H…N1 hydrogen-bonds, and those which show a tautomeric form in which N1 is protonated.











Similar content being viewed by others
References
Wagner W, Winkelmann E (1972) Arzneim -Forsch 22:1713
Klaymann DL, Bartosevich JF, Griffin TS, Mason CJ, Scovill JP (1979) J Med Chem 22:855. doi:10.1021/jm00193a020
Lewis A, Shepard RG (1970) In: Buerger AJ (ed) Medicinal chemistry. Wiley, NewYork, p 431
Malatesta P, Accinelli GP, Quaglia G (1959) Ann Chim Rome 49:397
Logan JC, Fox MP, Morgan JH, Makohon AM, Pfay CJJ (1975) Gen Virol 28:271
Lin LF, Lee SJ, Chen CT (1977) Heterocycles 7:347
Schultz TW, Ranney TS (1988) Toxicology 53:159
Dawson DA, Shultz TW, Baker LL, Mannar A (1990) J Appl Toxicol 10:59. doi:10.1002/jat.2550100111
Liu MC, Lin TS, Penketh P, Sartorelli AC (1995) J Med Chem 38:4234. doi:10.1021/jm00021a012
Ali A, Livingston SE (1974) Coord Chem Rev 13:101. doi:10.1016/S0010-8545(00)80253-2
Campbell MJM (1975) Coord Chem Rev 15:279. doi:10.1016/S0010-8545(00)80276-3
Padhye S, Kauffman GB (1985) Coord Chem Rev 63:127. doi:10.1016/0010-8545(85)80022-9
Casas JS, Garcia-Tasende MS, Sordo J (2000) Coord Chem Rev 209:197. doi:10.1016/S0010-8545(00)00363-5
West DX, Padhey SB, Sonawane PB, Kumbhar AS, Yernade RG (1993) Coord Chem Rev 123:49. doi:10.1016/0010-8545(93)85052-6
West DX, Padhye RG, Sonawane PB (1991) Struct Bond Berl 76:4
West DX, Carlson CS, Liberta AE, Scovill JP (1990) Transit Metab Chem 15:383. doi:10.1007/BF01177467
Maichle C, Castineiras A, Carballo R, Gebremedhin H, Lockwood MA, Ooms CE, Romack TJ, West DX (1995) Transit Metab Chem 20:228
West DX, Bain GA, Butcher RJ, Jasinski JP, Li Y, Pozdniakiv RY, Valdes-Martinez J, Toscano RA, Hernandez-Ortega S (1996) Polyhedron 15:665. doi:10.1016/0277-5387(95)00298-7
West DX, Lockwood MA, Castineiras A (1997) Transit Metab Chem 22:447. doi:10.1023/A:1018598810355
West DX, Owens MD (1998) Transit Metab Chem 23:87. doi:10.1023/A:1006966219867
Miller MCIII, Stineman CN, Vance JR, West DX, Hall IH (1999) Appl Organomet Chem 13:9. doi:10.1002/(SICI)1099-0739(199901)13:1<9::AID-AOC818>3.0.CO;2-#
Milczarska B, Foks H, Trapkowski Z, Milzynska-Kolaczek A, Janowiec M, Zwolska Z, Andrzejczyk Z (1998) Acta Pol Pharm Drug Res 5:289
Tian YP, Duan CY, Lu ZL, You XZ, Fun HK, Kandasamy S (1996) Polyhedron 15:2263. doi:10.1016/0277-5387(95)00477-7
Tian YP, Duan CY, Chao CY, You XZ, Zhang ZY, Mak TCW (1997) Inorg Chem 36:1247. doi:10.1021/ic9603870
Liu ZH, Duan CY, Hu J, You XZ (1999) Inorg Chem 38:1719. doi:10.1021/ic9711731
Valente EJ, Zubkowski JD, Jabalameli A, Mazhari S, Venkatraman R, Sullivan RH (1998) J Chem Crystallogr 28:27. doi:10.1023/A:1021722517880
Venkatraman R, Davis K, Shelby A, Zubkowski JD, Valente EJ (1999) J Chem Crystallogr 29:429. doi:10.1023/A:1009515111029
Venkatraman R, Ray PC, Fronczek FR (2004) Acta Crystallogr E60:m1035
Venkatraman R, Sweise R, Yamin BM (2005) Acta Crystallogr E61:o3914. doi:10.1107/S1600536805034409
Venkatraman R, Sitole L, Wilson MR, Fronczek FR (2006) Acta Crystallogr E62:m2992
Venkatraman R, Sitole L, Adams TD, Cameron JA, Fronczek FR (2007) Acta Crystallogr E63:m2212
Chambers CC, Archibong EF, Mazhari SM, Jabalameli A, Zubkowski JD, Sullivan RH, Valente EJ, Cramer CJ, Truhlar DG (1997) J Mol Struct Theochem 388:161
Kasper JS, Lonsdale K (eds) (1985) International tables for X-ray crystallography, vol IV. D. Reidel Publishing, Amsterdam
Sheldrick GM (1990) SHELXS (1986), Programs for solutions and refinement of crystal and molecular structures from X-ray diffraction data. Acta Crystallogr A46:467
Sheldrick G (1992) (SHELXL-93), crystallographic computing. Oxford University Press, Oxford
Wiles DM, Gingas BA, Suprunchuk T (1967) Can J Chem 45:469. doi:10.1139/v67-081
Bernstein J, Etter MC, Leiserowitz L (1994) In: Burgi HB, Dunitz JD (eds) Structure Correlation, vol 2, Chap. 11. VCH publishers, Weinheim
Zhou J, Wang YX, Chen CL, Li MX (2008) Acta Crystallogr E64:o94
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatraman, R., Ameera, H., Sitole, L. et al. Structures of Eight Thio(semi)carbazones Derived from 2-Acetylpyrazine, 2-Acetythiazole and Acetophenone. J Chem Crystallogr 39, 711–718 (2009). https://doi.org/10.1007/s10870-009-9541-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9541-0