Abstract
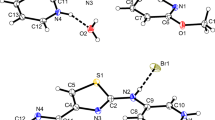
The synthesis of heterocyclic thioureas from heterocyclic amines with phenyl- or methylisothiocyanate or CS2 is described. Seven new X-ray crystal structures are reported: In N-(3-pyridyl)-N′-phenylthiourea (Pna21, a = 10.1453(3), b = 17.6183(5), c = 6.4787(2), V = 1158.02(6), Z = 4) hydrogen-bonding results in formation of a 3D network consisting of helices, which form channels parallel to the c-axis. In N-(4-pyridyl)-N′-phenylthiourea (P21/c, a = 16.9314(3), b = 10.3554(2), c = 13.5152(3), β = 106.5080(10), V = 2271.96(8), Z = 4, two independent molecules) hydrogen-bonding results in N–H···S bridged dimers and N–H···Py chains, forming a 2D sheet network. In N-(2-pyrimidyl)-N′-phenylthiourea (P21/c, a = 5.45900(10), b = 13.8559(2), c = 14.3356(3), β = 94.9800(10), V = 1080.24(3), Z = 4) and N-(2-pyrimidyl)-N′-methylthiourea (P21/c, a = 8.8159(5), b = 11.2386(5), c = 7.7156(4), β = 95.629(2), V = 760.76(7), Z = 4) pairs of intra- and intermolecular N–H···N interactions produce dimers. Dimer formation through N–H···S occurs for N-(2-thiazolyl)-N′-methylthiourea (C2/c, a = 17.9308(3), b = 7.78260(10), c = 10.8686(2), β = 105.3740(10), V = 1462.42(4), Z = 8). Two symmetrically disubstituted thioureas were examined: N,N′-bis(2-pyridyl)thiourea (Fdd2, a = 15.1859(2), b = 30.1654(3), c = 9.44130(10), V = 4324.95(8), Z = 16) forms intra- and intermolecular N–H···Py hydrogen-bonds, forming a 1D zigzag chain and N,N′-bis(3-pyridyl)thiourea (P21/c, a = 13.2461(2), b = 6.26170(10), c = 12.3503(2), β = 96.0160(10), V = 1018.73(3), Z = 4) forms intermolecular N–H···Py hydrogen-bonds, resulting in 2D sheets.
Index abstract
Hydrogen-Bonding Networks in Heterocyclic Thioureas.
Aakarsh Saxena and Robert D. Pike*
The synthesis and hydrogen bonded structures of various pyridyl-, pyrimidyl-, and thiazolyl-substituted thioureas is presented.












Similar content being viewed by others
References
(a) Tobe Y, Sasaki S-I, Mizuno M, Hirose K, Naemura, K (1998) J Org Chem 63:7481; (b) Succaw GL, Weakley TJR, Han F, Doxsee KM (2005) Cryst Growth Des 5:2288
(a) Yutronic M, Manriquez V, Jara P, Witke O, Merchán J, Gonzáles G (2000) J Chem Soc Perkin Trans 2 1757; (b) Babb JEV, Burke NJ, Burrows AD, Mahon MF, Slade DMK (2003) Cryst Eng Comm 5:226
Rudd MD, Lindeman SV, Husebye S (1997) Phosphorus Sulfur Silicon Relat Elem 123:313
Angelova O, Kossev K, Atanasov V (1999) Acta Crystallogr Sect C 55:220
(a) West DX, Hermetet AK, Ackerman LJ, Valdes-Martinez J, Hernandez-Ortega S (1999) Acta Crystallogr Sect C 55:811; (b) Valdes-Martinez J, Hernandez-Ortega S, West DX, Ackerman LJ, Swearingen JK, Hermetet AK (1999) J Mol Struct 478:219; (c) Kaminsky W, Goldberg KI, West DX (2002) J Mol Struct 605:9
Venkatachalam TK, Sudbeck E, Uckun FM (2004) J Mol Struct 687:45
Tellez F, Cruz A, Lopez-Sandoval H, Ramos-Garcia I, Gayosso M, Castillo-Sierra RN, Paz-Michel B, Noth N, Flores-Parra A, Contreras R (2004) Eur J Org Chem 4203
Tsogoeva SV, Hateley MJ, Yalalov DA, Meindl K, Weckbecker C, Huthmacher K (2005) Bioorg Med Chem 13:5680
Zhong H-P, Long L-S, Huang R-B, Zheng L-S, Ng SW (2003) Acta Crystallogr Sect C 59:o1596
Allen FH, Bird CM, Rowland RS, Raithby PR (1997) Acta Crystallogr Sect B 53:680
SAINT+ (2001) Bruker Analytical X-ray Systems. Madison, WI
SADABS (2001) Bruker Analytical X-ray Systems. Madison, WI
Sheldrick GM SHELXTL (2001) Crystallographic Computing System, Version 6.12, Bruker Analytical X-ray Systems. Madison, WI
Mercury 1.4.2 (2007) Cambridge Crystallographic Data Centre. Cambridge, UK
Hansen ET, Petersen HJ (1984) Synth Commun 14:537
Manley PW, Quast U (1992) J Med Chem 35:2327
Deady LW, Ganame D, Hughes AB, Quazi NH, Zanatta SD (2002) Aust J Chem 55:287
Fan Y, Lu H, Hou H, Zhou Z, Zhao Q, Zhang L, Cheng F (2000) J Coord Chem 50:65
Yamaguchi K, Shudo K (1991) J Agric Food Chem 39:793
Corbin PS, Zimmerman SC (2000) J Am Chem Soc 122:3779
Kumar DK, Jose DA, Das A, Dastidar P (2005) Chem Commun 4059
Acknowledgement
This research was supported in part by donors of the American Chemical Society Petroleum Research Fund (44891-B3). We are indebted to NSF (CHE-0443345) and the College of William and Mary for the purchase of the X-ray equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saxena, A., Pike, R.D. Hydrogen-Bonding Networks in Heterocyclic Thioureas. J Chem Crystallogr 37, 755–764 (2007). https://doi.org/10.1007/s10870-007-9246-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-007-9246-1