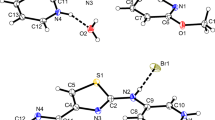
The crystal structures of the 1:1 proton-transfer compounds of 5-sulfosalicylic acid with the ortho-substituted monocyclic heteroaromatic Lewis bases, 2-aminopyridine, 2-hydroxypyridine and 2-aminopyrimidine, viz. 2-aminopyridinium 5-sulfosalicylate (1), 2-hydroxypyridinium 5-sulfosalicylate monohydrate (2) and 2-aminopyrimidinium 5-sulfosalicylate monohydrate (3) have been determined and their hydrogen-bonding patterns described. All compounds are monoclinic, space group P21/c, with Z=4 in cells with dimensions a=7.898(5), b=11.159(11), c=14.912(7) Å, β=96.849(11)° (1);=7.260(2), b=15.292(3), c=12.615(2) Å, β=102.45(5)° (2) and a=7.0430(7), b=12.1871(16), c=16.2825(12) Å, β=101.364(7)° (3). All three compounds show some molecular disorder, in 1 within the cation species and with both 2 and 3, a similar rotational disorder in the anion sulfonate group. Hydrogen bonding in all three compounds together with significant cation-anion or cation-cation inter-ring π–π interactions generate three-dimensional layered polymer structures.






Similar content being viewed by others
References
Attig, R.; Mootz, D. Acta Crystallogr. 1977, B 33, 2422.
Aliev, Z.G.; Atovmyan, L.L.; Ukshe, A.E. Zh. Strukt. Khim. 1995, 36, 947.
Attig, R.; Williams, J.M. J. Chem. Phys. 1977, 66, 1389.
Mootz, D.; Fayos, J. Acta Crystallogr. 1970, B 26, 2046.
Merschenz-Quack, A.; Mootz, D. Acta Crystallogr. 1990, C 46, 1478.
Bakasova, Z.B.; Abdybaliev, D.A.; Sharipov, Kh. T.; Akbaev, A.A.; Ibragimov, B.T.; Talipov, S.A.; Ismankulov, A.I. Uzb. Khim. Zh. 1991, pp. 22–25.
Smith, G.; Wermuth, U.D.; White, J.M. Acta Crystallogr. 2005, C 61, o105.
Smith, G.; Wermuth, U.D.; White, J.M. Acta Crystallogr. 2005, E 61, o313.
Smith, G. Acta Crystallogr. 2005, E 61, o3398.
Smith, G.; Wermuth, U.D.; Healy, P.C. Acta Crystallogr. 2006, E 62, o1863.
Smith, G.; Wermuth, U.D.; Healy, P.C. Acta Crystallogr. 2005, C 61, o555.
Muthiah, P.J.; Hemamalini, M.; Bocelli, G.; Cantoni, A. Acta Crystallogr. 2003, C 59, o2015.
Smith, G.; Wermuth, U.D.; White, J.M. Acta Crystallogr. 2004, C 60, o575.
Fan, S.-R.; Xiao, H.-P.; Zhu, L.-G. Acta Crystallogr. 2005, E 61, o253.
Madarasz, J.; Bombicz, P.; Jarmi, K.; Ban, M.; Pokol, G.; Gal, S. J. Therm. Anal. Calorim. 2002, 69, 281.
Raj, S.B.; Sethuraman, V.; Francis, S.; Hemamalini, M.; Muthiah, P.T.; Bocelli, G.; Cantoni, A.; Rychlewska, U.; Warzajtis, B. Cryst. Eng. Comm. 2003, 5, 70.
Hemamalini, M.; Muthiah, P.J.; Sridhar, B.; Rajaram, R.K. Acta Crystallogr. 2005, E 61, o1480.
Zhang, X.-L.; Chen, X.-M.; Ng, S.W. Acta Crystallogr. 2004, E 60, o453.
Gao, S.; Huo, L.-H.; Ng, S.W. Acta Crystallogr. 2004, E 60, o2197.
Smith, G.; Wermuth, U.D.; Healy, P.C. Acta Crystallogr. 2004, E 60, o687.
Etter, M.C.; Adsmond, D.A. J. Chem. Soc., Chem. Commun. 1990, 589.
Lynch, D.E.; Smith, G.; Freney, D.; Byriel, K.A.; Kennard, C.H.L. Aust. J. Chem. 1994, 47, 1097.
Smith, G.; Gentner, J.M.; Lynch, D.E.;.Byriel, K.A.; Kennard, C.H.L. Aust. J. Chem. 1995, 48, 1151.
Byriel, K.A.; Kennard, C.H.L.; Lynch, D.E.; Smith, G.; Thompson, J.G. Aust. J. Chem. 1992, 45, 969.
Lynch, D.E.; Latif, T.; Smith, G.; Byriel, K.A.; Kennard, C.H.L. J. Chem. Crystallogr. 1997, 27, 567.
Smith, G.; Bott, R.C.; Wermuth, U.D. Acta Crystallogr. 2000, C 56, 1505.
Buyükgüngör, O.; Odabasoglu, M.; Albayrak, C.; Lönnecki, P. Acta Crystallogr. 2004, C 60, o470.
Haynes, D.A.; Chisholm, J.A.; Jones, W.; Motherwell, W.D.S. Cryst. Eng. Comm. 2004, 6, 584.
Allen, F.H.; Raithby, P.R.; Shields, G.P.; Taylor, R. Chem. Commun. 1998, 1034.
Altomare, A.; Cascarno, G.; Giocovasso, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. J. Appl. Crystallogr. 1994, 27, 435.
Sheldrick, G.M.: SHELXL 97: Program for Crystal Structure Refinement, University of Göttingen, Germany.
TeXsan for Windows: Structure Analysis Software. Version 1.06, 1999. Molecular Structure Corporation, New Trails Drive, The Woodlands, TX77381, USA.
Spek, A.L. PLATON: A Multipurpose Crystallographic Tool. J. Appl. Crystallogr. 2003, 36, 7.
Acknowledgments
The authors acknowledge financial support from the School of Physical and Chemical Sciences (Queensland University of Technology) and the School of Science, Griffith University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary material
CCDC 286383, 286384 and 286385 contain supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/datarequest/cif by e-mailing datarequest@ccdc.cam.ac.uk, or contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ.
Rights and permissions
About this article
Cite this article
Smith, G., Wermuth, U.D. & Healy, P.C. Hydrogen bonding in proton-transfer compounds of 5-sulfosalicylic acid with ortho-substituted monocyclic heteroaromatic Lewis bases. J Chem Crystallogr 36, 841–849 (2006). https://doi.org/10.1007/s10870-006-9122-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-006-9122-4