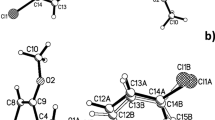
1-[2-(p-Tolyl)-1-diazenyl]-3-({3-[2-(p-tolyl)-1-diazenyl]perhydrobenzo[d]imidazol-1-yl}methyl)perhydrobenzo[d]imidazole(1) has been synthesized by reactionof a mixture of 1,2-diaminocyclohexane and formaldehyde withp-toluene diazonium chloride inaqueous solution. The product has been characterized by IR and NMRspectroscopy and elemental analysis. A crystal grown from solution ina mixed solvent system of ethyl acetate and hexanes was analyzed byX-ray crystallography. The solution of the crystal structure of(1) is important in establishing theconnectivity of this molecule and other compounds of similarstructure. The crystal structure of (1) is compared with the previously reportedstructure of the p-cyano analogue(2). Compounds (1) and (2)differ principally in the relative orientation of the heterocyclicrings; in (1), the molecule has adistinct V-shape, whereas compound (2) adopts a more extended conformation.Significant conjugation within the triazene moieties is evident inboth (1) and (2), as manifested in the N465=N andN–N bond lengths. The conjugation is greater in (2) due to the extended conjugation through tothe nitrile group. The title compound (1){C29H40N8}crystallizes in the monoclinic, space group C2/c, with lattice constants: a = 30.532(6) Å,b =5.9050(12) Å, c= 15.463(3) Å, α = 90°,β = 99.94(3)°, γ = 90°,V =2746.0(10) Å3, Z = 4, D c =1.209 mg m−3,F(000) = 1076, R 1 = 0.0785,wR 2= 0.1877.



Similar content being viewed by others
References
Glister,J.F.; Vaughan, K.; Bertolasi, V. J. Chem.Crystallogr. 2004,34, 175.
Vaughan, K. Org. Prep. Proc. Int. 2001, 33,59.
Peori, M.B.; Vaughan, K.; Hooper, D.L. J. Org. Chem. 1998, 63, 7437.
Biradha, K.; Peori, M.B.; Vaughan, K.; Zaworotko, M.J. J. Chem. Crystallogr. 1999, 29, 145.
Moser, S.;Church, R.; Peori, M.B.; Vaughan, K. Can. J.Chem. 2005, 83, 1071.
Peori,M.B.; Vaughan, K.; Bertolasi, V. J. Chem.Crystallogr. 2005,35, 297.
Glister, J.F.; Vaughan, K. J. Heterocycl. Chem. 2006, 43, 217.
Sheldrick, G.M. SADABS, University of Göttingen,Germany, 1996.
Sheldrick, G.M. SHELXTL, Release 5.03, SiemensAnalytical X-ray Instruments, Inc., Madison, WI,1994.
Johnson, C.K.ORTEP II, Report ORNL-5138; Oak Ridge National Laboratory, Oak Ridge,Tennessee, 1976.
Barra, M.; Srivastava, S.; Brockman, E. J. Phys. Org. Chem. 2004, 17,1057; Golding, B.T.; Kemp, T.J.; Narayanaswamy, R.; Waters, B.W.J. Chem. Res. (S), 1984, 130.
Biradha,K.; Singer, R.D.; Stark, A.; Vaughan, K.; Zaworotko, M.J. J. Chem. Crystallogr. 1998, 28,797.
Acknowledgments
The authors are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support (to HJ and KV) and to theStudent Employment Experience Program (SEEP) at Saint Mary'sUniversity for a Summer Research Award to Julie Glister. We are alsograteful to the Atlantic Region Magnetic Resonance Centre atDalhousie University for providing NMRspectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementarymaterial
Crystallographic data for structuralanalysis reported in this paper have been deposited with theCambridge Crystallographic Data Centre and allocated the depositionnumber CCDC 246453. These data can be obtained free of charge viawww.ccdc.cam.ac.uk/conts/retrieving.html or onapplication to CCDC, Union Road, Cambridge CB2 1EZ, UK [fax:(+44)1223-336033, e-mail:deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Glister, J.F., Jenkins, H. & Vaughan, K. 1-[2-(p-Tolyl)-1-diazenyl]-3-({3-[2-(p-tolyl)-1-diazenyl] perhydrobenzo[d]imidazol-1-yl}methyl)perhydrobenzo[d]imidazole: synthesis,characterization and X-ray crystal structure. J Chem Crystallogr 36, 445–452 (2006). https://doi.org/10.1007/s10870-005-9051-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-005-9051-7