Abstract
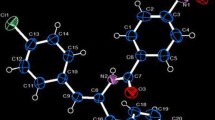
The title compound 5-bromo-1-(2-cyano-pyridin-4-yl)-1H-indazole-3-carboxylic acid diethylamide, C18H16BrN5O, is prepared from 5-bromoindazole-3-carboxylic acid methylester. N 1-arylation is carried out with 4-chloro-2-cyanopyridine and the resulting product is converted to diethylamide by reacting with thionyl chloride and diethylamine. The structure is identified from its FT-IR, 1H NMR, 13C NMR spectroscopy, elemental analysis data and unambiguously confirmed by single crystal X-ray diffraction studies. There are two symmetry independent molecules in the asymmetric unit with no significant differences in bond lengths and angles. The title compound crystallizes in the triclinic system, space group \(P\bar 1\), with a = 11.2330(2); b = 11.6130(2); c = 15.4710(3) Å, α = 92.515(1)°; β = 109.956(1)°; γ = 107.199(1)°; V = 1788.45(6)Å3 and z = 4. An intramolecular C-H…N hydrogen bond forms an S(6) ring motif in one of the unique molecules. In the crystal, two molecules are linked about a center of inversion by C-H…O hydrogen bonded dimers generating an R 22 (16) ring motif. The crystal packing is stabilized by C-H…N, C-H…O hydrogen bonds and π…π stacking interactions.
Similar content being viewed by others
References
A. M. Maharramov, A. I. Ismiyev, and B. A. Rashidov, Acta Crystallogr. E 67, o480 (2011).
N. El Brahmi, M. Benchidmi, E. M. Essassi, et al., Acta Crystallogr. E 68, o3368 (2012).
H. Cerecetto, A. Gerpe, M. Gonzalez, V. J. Arvan, and C. O. Ocariz, Mini-Rev. Med. Chem. 5, 869 (2005).
L. Mosti, G. Menozzi, P. Fossa, P. Schenone, E. Lampa, C. Parrillo, M. D’Amisco, and F. Rossi, Farmaco 47, 567 (1992).
A. J. Soures, J. Gao, D. Wodka, A. S. Judd,, M. M. Mulhern, J. J. Napier, M. E. Brune, E. N. Bush, S. J. Brodjian, B. D. Dayton, R. Shapiro, L. E. Hernadez, K. C. Marsh, H. L. Sham, and C. A. Collins Bioorg. Med. Chem. Lett. 15, 2752 (2005).
A. Gerpe, G. Aguirre, L. Boiani, H. Cerecetto, M. Gonzalez, C. Olea-Aar, C. Rigol, J. D. Maya, A. Morello, O. E. Piro, V. J. Aran, A. Azqueta, A. L. Cerian, A. Monge, M. A. Rojas, and G. Yaluff, Bioorg. Med. Chem. 14, 3467 (2006).
M. Boehringer, H. J. Boehm, D. Bur, H. Gmuender, W. Huber, W. Klaus, D. Kostrewa, H. Kuehne, T. Luebbers, N. Meunier-Keller, and F. Mueller, J. Med. Chem. 43, 2664 (2000).
J. D. Rodgers, B. C. Cordova, H. Wang, S. Erickson-Viitanen, R. M. Klabe, L. Bacheler, B. C. Cordova, and H. C. Chang, Bioorg. Med. Chem. Lett. 8, 715 (1998).
M. Iwakubo, A. Takami, Y. Okada, T. Kawata, Y. Tagami, H. Ohashi, M. Sato, T. Sugiyama, K. Fukushima, and H. Iijima, Bioorg. Med. Chem. Lett. 15, 350 (2007).
J. H. Ko, S. W. Yeon, J. S. Ryu, T. Y. Kim, E. H. Song, H. J. You, R. E. Park, and C. K. Tyu, Bioorg. Med. Chem. Lett. 16, 6001 (2006).
M. Burger, H. Rehwinkel, H. Schaecke, M. Lepisto, and K. Edmann, WO2008/052808.
R. J. Butcher, M. Akkurt, S. Samshuddin, B. Narayana, and H. S. Yathirajan, Acta Crystallogr. E 67, o1346 (2011).
L. Long, B.-N. Liu, M. Liu, and D.-K. Liu, Acta Crystallogr. E 67, o1546 (2011).
APEX2, SAINT-PLUS, and SADABS (Bruker AXS Inc., Madison, Wisconsin, 2004).
G. M. Sheldrick Acta Crystallogr. A 64, 112 (2008).
C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, and P. A. Wood, J. Appl. Crystallogr. 41, 466 (2008).
L. J. Farrugia, J. Appl. Crystallogr. 32, 837 (1999).
J. C. Antilla, J. M. Baskin, T. E. Barder, and S. L. Buchwald, J. Org. Chem. 69, 5578 (2004).
J. Bernstein, R. E. Davis, L. Shimoni, and N.-L. Chang, Angew. Chem. Int. Ed. Engl. 34, 1555 (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Anuradha, G., Vasuki, G., Surendrareddy, G. et al. Synthesis, spectroscopic characterization and crystal structure of 5-bromo-1-(2-cyano-pyridin-4-yl)-1H-indazole-3-carboxylic acid diethylamide. Crystallogr. Rep. 59, 527–535 (2014). https://doi.org/10.1134/S1063774514040026
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774514040026