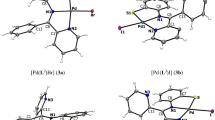
The three-dimensional molecular and crystal structures of a pair of enantiopure mirror complexes of Ni (II) to chiral Schiff bases of (R)- and (S)-serine with (S)- and (R)-2-N-(N′-benzylprolyl)-aminobenzophenone (BPB), denoted as (S,R)-BPB-Ser-Ni and (R,S)-BPB-Ser-Ni, respectively, have been determined by X-ray diffraction. The complexes crystallize in the orthorhombic space group P2 1 2 1 2 1 with unit cell parameters: a = 10.050(2) Å, b = 10.100(2) Å, c = 26.514(5) Å, V = 2691.3(9) Å3, and Z = 4 for (S,R)-BPB-Ser-Ni; and a = 9.878(2) Å, b = 10.130(2) Å, c = 26.304(5) Å, V = 2632.1(9) Å3, and Z = 4 for (R,S)-BPB-Ser-Ni. The Ni atom is square-planar-coordinated to N(1), N(2), O(4), and N(3) of the Schiff base with minor distortion. The hydroxymethyl group on the serine moiety is axial to the coordination plane and parallel to the neighboring phenyl group.



Similar content being viewed by others
References
Belokon, Y.N.; Zeltzer, I.E.; Bakhmutov, V.I.; Saporovskaya, M.B.; Ryzhov, M.G.; Yanovsky, A.I.; Struchkov, Y.T.; Belikov, V.M. J. Am. Chem. Soc. 1983, 105, 2010–2017.
Belokon, Y.N.; Bulychev, A.G.; Vitt, S.V.; Struchkov, Y.T.; Batsanov, A.S.; Timofeeva, T.V.; Tsyryapkin, V.A.; Ryzhov, M.G.; Lysova, L.A.; Bakhmutov, V.I.; Belikov, V.M. J. Am. Chem. Soc. 1985, 107, 4252–4259.
Williams, R.M. Synthesis of Optically Active α-Amino Acids, Pergamon Press: Oxford, 1989.
Nájera, C. Synlett 2002, 9, 1388–1403.
Li, Z.; Wan, H.; Wei, P.; Shi, Y.; Ouyang, P. Chin. J. Org. Chem. 2005, 25, 881–892.
Belokon, Y.N.; Bakhmutov, V.I.; Chernoglazova, N.I.; Kochetkov, K.A.; Vitt, S.V.; Garbalinskaya, N.S.; Belikov, V.M. J. Chem. Soc., Perkin Trans. 1 1988, 305–312.
Belokon, Y.N.; Sagyan, A.S.; Djamagaryan, S.A.; Bakhmutov, V.I.; Belikov, V.M. Tetrahedron 1988, 44, 5507–5541.
Belokon, Y.N.; Sagyan, A.S.; Djamagaryan, S.A.; Bakhmutov, V.I.; Vitt, S.V.; Batsanov, A.S.; Struchkov, Y.T.; Belikov, V.M. J. Chem. Soc., Perkin Trans. 1 1990, 2301–2310.
Kukhar, V.P.; Belokon, Y.N.; Soloshonok, V.A.; Svistunova, N.Y.; Rozhenko, A.B.; Kuzmina, N.A. Synthesis 1993, 117–120.
Belokon, Y.N.; Kochetkov, K.A.; Ikonnikov, N.S.; Strelkova, T.V.; Harutyunyan, S.R.; Saghiyan, A.S. Tetrahedron: Asymmetry 2001, 12, 481–485.
Larionov, L.V.; Saveleva, T.F.; Kochekov, K.A.; Ikonnokov, N.S.; Kozhushkov, S.I.; Yufit, D.S.; Howard, J.A.K.; Khrustalev, V.N.; Belokon, Y.N. de Meijere, A. Eur.J. Org. Chem. 2003, 5, 869–877.
Saghiyan, A.S.; Geolchanyan, A.V.; Petrosyan, S.G.; Ghochikyan, T.V.; Haroutunyan, V.S.; Avetisyan, A.A.; Belokon, Y.N.; Fisher, K. Tetrahedron: Asymmetry 2004, 15, 705–711.
Belokon, Y.N. Pure Appl. Chem. 1992, 64, 1917–1924.
Belokon, Y.N. Janssen Chim. Acta 1992, 10(2), 4–12.
Soloshonok, V.A.; Kukhar, V.P.; Galushko, S.V.; Svistunova, N.Y.; Avilov, D.V.; Kuzmina, N.A.; Raevski, N.I.; Struchkov, Y.T.; Pysarevsky, A.P.; Belokon, Y.N. J. Chem. Soc., Perkin Trans. 1 1993, 3143–3155.
Soloshonok, V.A.; Avilov, D.V.; Kukhar, V.P.; Tararov, V.I.; Saveleva, T.F.; Churkina, T.D.; Ikonnikov, N.S.; Kochetkov, K.A.; Orlova, S.A.; Pysarevsky, A.P.; Struchkov, Y.N.; Raevsky, N.I.; Belokon, Y.N. Tetrhedron: Asymmetry 1995, 6, 1741–1756.
Soloshonok, V.A.; Avilov, D.V.; Kukhar, V.P. Tetrahedron 1996, 38, 12433–12442.
Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 4th Ed. Butterworth Heinemann: Oxford, 1997.
Belokon, Y.N.; Tararov, V.I.; Maleev, V.I.; Saveleva, T.F.; Ryzhov, M.G. Tetrahedron: Asymmetry 1998, 9, 4249–4252.
Sheldrick, G.M. SHELXS-97 and SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen: Germany, 1997.
Sheldrick, G.M. Acta Crystallogr. 1990, A46, 467–473.
Farrugia, L.J. J. Appl. Crystallogr. 1997, 30, 565.
Acknowledgments
We thank the Hi-Tech R & D Program of China (Grant No.2003AA235051) and the Major Basic R & D Program of China (Grant No.2003CB716001) for financial support. The corresponding author Z. Li thanks Prof. Yuri N. Belokon with A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences for kindly help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, W., Cao, F., Li, Z. et al. Synthesis and crystal structures of complexes of Ni (II) to chiral Schiff bases of (R)- and (S)-serine with (S)- and (R)-2-N-(N′-benzylprolyl)-aminobenzophenone. J Chem Crystallogr 36, 61–65 (2006). https://doi.org/10.1007/s10870-005-9025-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-005-9025-9