Abstract
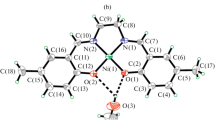
Two new mononuclear complexes, [NiL1] · CH3OH (I) and [NiL2] (II), have been prepared from the tetradentate Schiff bases N,N'-bis(5-methylsalicylidene)ethylenediamine (H2L1) and N,N'-bis(5-methylsalicylidene)- o-phenylenediamine (H2L2), respectively. The complexes have been characterized by physico-chemical and spectroscopic methods, as well as single-crystal X-ray determination (CIF files nos. 1428969 (I), 1428968 (II)). Complex I crystallizes in the triclinic space group P1 with a = 6.7387(14), b = 10.7010(17), c = 12.681(2) Å, α = 87.059(2)°, β = 88.828(2)°, γ = 89.901(2)°, V = 913.0(3) Å3, Z = 2. Complex II crystallizes in the monoclinic space group P21/n with a = 12.1437(11), b = 8.0537(8), c = 18.4545(18) Å, β = 105.088(2)°, V = 1742.7(3) Å3, Z = 4. The nickel atoms in the complexes are coordinated by two phenolate O and two imine N atoms of the tetradentate Schiff base ligands, forming square planar coordination. The complexes and the Schiff base compounds were assayed for antibacterial activities against three Gram-positive bacterial strains (B. subtilis, S. aureus, and St. faecalis) and three Gram-negative bacterial strains (E. coli, P. aeruginosa, and E. cloacae) by MTT method. As a result, the complexes showed effective antimicrobial activity against the microorganisms tested.
Similar content being viewed by others
References
Zhang, J.-C., Li, Y.-N., Huang, D., et al., Chin. J. Inorg. Chem., 2014, vol. 30, no. 2, p. 425.
Niu, F., Yan, K.-X., Pang, L.H., et al., Inorg. Chim. Acta, 2015, vol. 435, p. 299.
Mohamed, R.G., Elantabli, F.M., Helal, N.H., et al., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2015, vol. 45, no. 12, p. 1839.
Matin, S.J. and Khojasteh, R.R., Russ. J. Coord. Chem., 2015, vol. 85, no. 7, p. 1763.
Li, W., Ding, B.-W., Sun, H., et al., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2012, vol. 42, no. 5, p. 666.
Hazari, P.P., Pandey, A.K., Chaturvedi, S., et al., Chem. Biol. Drug Des., 2012, vol. 79, no. 2, p. 223.
Ramadan, R.M., Abu Al-Nasr, A.K., and Noureldeen, A.F.H., Spectrochim. Acta, A, 2014, vol. 132, p. 417.
Khoo, T.-J., Bin Break, M.K., Crouse, K.A., et al., Inorg. Chim. Acta, 2014, vol. 413, p. 68.
Mukherjee, T., Pessoa, J.C., Kumar, A., et al., Dalton Trans., 2013, vol. 42, no. 7, p. 2594.
Maurya, R.C., Malik, B.A., Mir, J.M., et al., J. Coord. Chem., 2015, vol. 68, no. 16, p. 2902.
Lu, Y., Shi, D.-H., You, Z.-L., et al., J. Coord. Chem., 2012, vol. 65, no. 2, p. 339.
Zhou, X.-S., Cheng, X.-S., Li, Y.-N., et al., Chin. J. Inorg. Chem., 2013, vol. 29, no. 2, p. 397.
Sheldrick, G.M., SAINT (version 6.02), SADABS (version 2.03), Madison: Bruker AXS lnc., 2002.
Sheldrick, G.M., SHELXL-97, Program for Crystal Structure Solution, Göttingen: Univ. of Göttingen, 1997.
Meletiadis, J., Meis, J.F., Mouton, J.W., et al., J. Clin. Microbiol., 2000, vol. 38, no. 8, p. 2949.
Geary, W.J., Coord. Chem. Rev., 1971, vol. 7, no. 1, p. 81.
Lal, R.A., Choudhury, S., Ahmed, A., et al., J. Coord. Chem., 2009, vol. 62, no. 23, p. 3864.
Surati, K. and Thaker, B.T., Spectrochim. Acta, A, 2010, vol. 75, no. 1, p. 235.
Jana, A., Majumder, S., Carrella, L., et al., Inorg. Chem., 2010, vol. 49, no. 19, p. 9012.
Rusere, L.N., Shalumova, T., Tanski, J.M., et al., Polyhedron, 2009, vol. 28, no. 17, p. 3804.
Prabhakar, M., Zacharias, P.S., and Das, S.K., Inorg. Chem. Commun., 2006, vol. 9, no. 9, p. 899.
Abe, Y., Akao, H., Yoshida, Y., et al., Inorg. Chim. Acta, 2006, vol. 359, no. 10, p. 3147.
Taherlo, R. and Salehi, M., Inorg. Chim. Acta, 2014, vol. 418, p. 180.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Xu, Y., Xue, L. & Wang, Z.G. Synthesis, X-ray crystal structures, and antibacterial activities of Schiff base nickel(II) complexes with similar tetradentate Schiff bases. Russ J Coord Chem 43, 314–319 (2017). https://doi.org/10.1134/S1070328417050098
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328417050098