Abstract
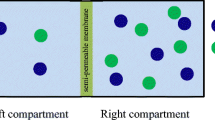
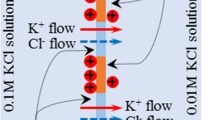
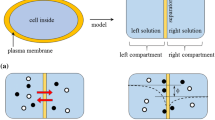
Donnan theory and Goldman-Hodgkin-Katz equation (GHK eq.) state that the nonzero membrane potential is generated by the asymmetric ion distribution between two solutions separated by a semipermeable membrane and/or by the continuous ion transport across the semipermeable membrane. However, there have been a number of reports of the membrane potential generation behaviors in conflict with those theories. The authors of this paper performed the experimental and theoretical investigation of membrane potential and found that (1) Donnan theory is valid only when the macroscopic electroneutrality is sufficed and (2) Potential behavior across a certain type of membrane appears to be inexplicable on the concept of GHK eq. Consequently, the authors derived a conclusion that the existing theories have some limitations for predicting the membrane potential behavior and we need to find a theory to overcome those limitations. The authors suggest that the ion adsorption theory named Ling’s adsorption theory, which attributes the membrane potential generation to the mobile ion adsorption onto the adsorption sites, could overcome those problems.















Similar content being viewed by others
References
Ling, G.N.: A Revolution in the Physiology of the Living Cell. Krieger Publishing Co., Malabar, Florida (1992)
Ling, G.N.: Life at the cell and below-cell level: the hidden history of a fundamental revolution Biology. Pacific Press, New York (2001)
Chou, T.-J., Tanioka, A.: Ionic behavior across charged membranes in methanol-water solutions. 1: membrane potential. J. Membrane Sci. 144, 275–284 (1998)
Wasserman, E., Felmy, A.: Computation of the electrical double layer properties of semipermeable membranes in multicomponent electrolytes. Appl. Environ. Microbiol. 64, 2295–2300 (1998)
Kodzwa, M.G., Staben, M.E., Rethwisch, D.G.: Photoresponsive control of ion-exchange in leucohydroxide containing hydrogel membranes. J. Membrane Sci. 158, 85–92 (1999)
Chou, T.-J., Tanioka, A.: Membrane potential of composite bipolar membrane in ethanol-water solutions: the role of the membrane interface. J. Colloid Interface Sci. 212, 293–300 (1999)
Jauas, T., Jauas, T., Krajiriski, H.: Membrane transport of polysialic acid chains: modulation of transmembrane potential. Eur. Biophys. J. 29, 507–514 (2000)
Jeong, S., Lee, W., Yang, W.: Non stationary ionic current through polymer charged membrane. Bull. Korean Chem. Soc. 24, 937–942 (2003)
Davis, T.A., Yezek, L.P., Pinheiro, J.P., van Leeuwen, H.P.: Measurement of Donnan potentials in gels by in situ microelectrode voltammetry. J. Electroanal. Chem. 584, 100–109 (2005)
Chen, C.-C., Derylo, M.A., Baker, L.A.: Measurement of ion currents through porous membranes with scanning ion conductance microscopy. Anal. Chem. 81, 4742–4751 (2009)
Das, S.: Explicit interrelationship between Donnan and surface potentials and explicit quantification of capacitance of charged soft interfaces with pH-dependent charge density. Colloids Surf. A Physicochem. Eng. Asp. 462, 69–74 (2014)
Colacicco, G.: Electrical potential at an oil/water interface. Nature 207, 936–938 (1965)
Colacicco, G.: Reversal of potential across a liquid non-aqueous membrane with regard to membrane excitability. Nature 207, 1045–1047 (1965)
Tamagawa, H., Morita, S.: Membrane potential generated by ion adsorption. Membranes (online journal) 4, 257–274 (2014)
Ling, G.N.: K+ Localization in muscle cells by autoradiography, and identification of K+ adsorbing sites in living muscle cells with uranium binding sites in electron micrographs of fixed cell preparations. Physiol. Chem. Phys. 9, 319–327 (1977)
Ling, G.N.: Oxidative phosphorylation and mitochondrial physiology: a critical review of chemiosmotic theory, and reinterpretation by the association-induction hypothesis. Physiol. Chem. Phys. 13, 31–96 (1981)
Ling, G.N.: Truth in basic biomedical science will set future mankind free. Physiol. Chem. Phys. Med. NMR 41, 19–48 (2011)
Tamagawa, H.: Membrane potential generation without ion transport. Ionics 21, 1631–1648 (2014)
Tamagawa, H., Takahashi, Y.: Adhesion force behavior between two gels attached with an electrolytic polymer liquid. Mater. Chem. Phys. 107, 164–170 (2008)
Donnan, F.G.: Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. Zeitschrift Ftir Elektrochemie und Angewandte Physikalische Chemie 1, 572–581 (1911)
Donnan, F.G.: Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. J. Membr. Sci. 100, 45–55 (1995)
Ohmine, I., Tanaka, T.: Salt effects of the phase transition of ionic gels. J. Chem. Phys. 77, 5725–5729 (1982)
Ricka, J., Tanaka, T.: Swelling of ionic gels: quantitative performance of the donnan theory. Macromolecules 17, 2916–2921 (1984)
Ikeda, S., Kumagaia, H., Sakiyamaa, T., Chua, C.-H., Nakamura, K.: Method for analyzing pH-sensitive swelling of amphoteric hydrogels? Application to a polyelectrolyte complex gel prepared from Xanthan and Chitosan?. Biosci. Biotech. Biochem. 59, 1422–1427 (1995)
Norouzi, H.R., Azizpour, H., Sharafoddinzadeh, S., Barati, A.: Equilibrium swelling study of cationic Acrylamide-Based hydrogels: effect of synthesis parameters, and phase transition in polyelectrolyte solutions. J. Chem. Petroleum Engineer. 45, 13–25 (2011)
Mentré, P.: Saibou no naka no mizu (Water in the cell) (Japanese translation of L’eau dans la cellule, Masson, Paris, 1996). University Tokyo Press, Tokyo (2006)
Chang, D.C.: A physical model of nerve axon - I. Ionic distribution, potential profile, and resting potential. Bull. Math. Biol. 39, 2–22 (1977)
Wnek, G.E.: Perspective: do macromolecules play a role in the mechanisms of nerve stimulation and nervous transmission?. J. Polym. Sci. Polym. Phys. 54, 7–14 (2016)
Acknowledgments
The authors would like to express our gratitude to The MIKIYA Science And Technology Foundation for the financial support for conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Appendices
Appendix A
The left expression of (28) is derived by the equilibrium equation, COOK ⇌ COO− + K+, where K D is the dissociation constant. The left expression of (28) is transformed into the right expression of (28) using [Carboxyl] ≡ [COOK] + [COO−]. For the hydrogel synthesis, we used 1.44 g acrylic acid (M w = 72.06) [19]. Assuming that the volume of hydrogel synthesized was the same as the volume of deionized water used for the gel synthesis (We used 0.05 L of deionized water.), and using the experimentally measured swelling ratio, 20.80 (see Table 2), [Carboxyl] in G-0.0001 is given by (1.44 ÷ 72.06) ÷ (0.05 ×20.80) = 0.019 M = 19 molm−3. The macroscopic electroneutrality represented by (29) establishes at \(\mathrm {x} = +\infty \) under the condition [H+] and [OH−] are negligibly low.
Employing Donnan theory and \(\phi _{g}|_{x=+\infty }= -0.076\) V in Table 2, (30) is derived, where B ≡ e/kT. (30) gives \([\mathrm {K}^{+}(\mathrm {x}=+\infty )] = 2.02~\text {mol m}^{-3}\) and \([\text {Cl}^{-}(\mathrm {x}=+\infty )] = 0.005~\text {molm}^{-3}\). Using them and (29), \([\text {COO}^{-}]|_{x =+\infty } = 2.015\) molm−3 is obtained. Plugging [Carboxyl] = 19 molm−3, \([\mathrm {K}^{+}(\mathrm {x}=+\infty )] = 2.02~\text {mol m}^{-3}\) and \([\text {COO}^{-}]\mid _{x =+\infty } = 2.015\) molm−3 into the right expression of (28), K\(_{D} = 0.24~\text {molm}^{-3}\) is obtained.
Appendix B
K A in (19) was estimated by the following experiment: A 2 mm-thick hydrogel containing -SO3H groups was synthesized. The pregel solution consisted of 2-acrylamido-2-methylpropane sulfonic acid (20.7 g), N,N’-methylenebisacrylamide (0.077 g), N,N,N’,N’-tetra-methylethylenediamin (a few drops), ammonium persulfate (0.04g) and deionized water (50 g). The hydrogel synthesized was equilibrated in 0.01 M KCl solution. We assume that -SO3H was converted into -SO3K. We made measurement of potential, ψ, at the far inside of this hydrogel in reference to the bathing solution and observed ψ = 0.0165 V.
[K+] and [Cl−] at the far inside of the hydrogel is given by (31), where B ≡ e/kT. Because of the equilibrium equation SO\(_{3}^{-}\) + K+ \(\rightleftharpoons \) SO3K, (32) is derived, where K a is the association constant of -SO3K of hydrogel.
The total quantity of SO3K + SO\(_{3}^{-}\) is given by 20.7 ÷ 207 = 0.1 mol, where the molecular weight of 2-acrylamido-2-methylpropane sulfonic acid is 207. Assuming that the volume of gel synthesized was same as the volume of deionized water used for this synthesis, i.e. 0.05 L, and using the experimental swelling ratio of this hydrogel in 0.01 M KCl solution, 47, the total concentration of SO3K + SO\(_{3}^{-}\) in the hydrogel was given by 0.1 ÷ (0.05 ×47) = 0.043 M = 43 molm−3. Therefore, [Sulfonic] ≡ [SO\(_{3}^{-}\)] + [SO3K] = 43 molm−3. (32) is rearranged into (33) using [Sulfonic] = [SO\(_{3}^{-}\)] + [SO3K]. It is quite natural to believe that the electroneutrality is established at the far inside of hydrogel. Therefore, (34) establishes under the condition that [H+] and [OH−] are negligibly low. Using (31) and (34), [SO\(_{3}^{-}\)] at the far inside of hydrogel is given 13.7 molm−3. Therefore, using (31), (33), [Sulfonic] = 43 molm−3 and [SO\(_{3}^{-}\)] = 13.7 molm−3, K a is given 0.11 mol−1 m 3. Selemion CMV contains -SO3H groups, and the hydrogel so far described contains -SO3H groups, too. Therefore, we employed the K a of hydrogel as K A of the Selemion CMV.
Rights and permissions
About this article
Cite this article
Tamagawa, H., Ikeda, K. Generation of membrane potential beyond the conceptual range of Donnan theory and Goldman-Hodgkin-Katz equation. J Biol Phys 43, 319–340 (2017). https://doi.org/10.1007/s10867-017-9454-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-017-9454-7