Abstract
Image correlation spectroscopy (ICS) has been widely used to quantify spatiotemporal distributions of fluorescently labelled cell membrane proteins and receptors. When the membrane proteins are randomly distributed, ICS may be used to estimate protein densities, provided the proteins behave as point-like objects. At high protein area fraction, however, even randomly placed proteins cannot obey Poisson statistics, because of excluded area. The difficulty can arise if the protein effective area is quite large, or if proteins form large complexes or aggregate into clusters. In these cases, there is a need to determine the correct form of the intensity correlation function for hard disks in two dimensions, including the excluded area effects. We present an approximate but highly accurate algorithm for the computation of this correlation function. The correlation function was verified using test images of randomly distributed hard disks of uniform intensity convolved with the microscope point spread function. This algorithm can be readily modified to compute exact intensity correlation functions for any probe geometry, interaction potential, and fluorophore distribution; we show how to apply it to describe a random distribution of large proteins labeled with a single fluorophore.





Similar content being viewed by others
References
St-Pierre, P.R., Petersen, N.O.: Average density and size of microclusters of epidermal growth factor receptors on A432 cells. Biochem. 31, 2459–2463 (1992)
Petersen, N.O., Höddelius, P.L., Wiseman, P.W., Seger, O., Magnusson, K.E.: Quantitation of membrane receptor distributions by image correlation spectroscopy: concept and application. Biophys. J. 65, 1135–1146 (1993)
Petersen, N.O., Brown, C., Kaminski, A., Rocheleau, J., Srivastava, M., Wiseman, P.W.: Analysis of membrane protein cluster densities in situ by image correlation spectroscopy. Faraday Discuss. 111, 289–305 (1998)
Costantino, S., Comeau, J.W.D., Kohlin, D.L., Wiseman, P.W.: Accuracy and dynamic range of spatial image correlation and cross-correlation spectroscopy. Biophys. J. 89, 1251–1260 (2005)
Kohlin, D.L., Costantino, S., Wiseman, P.W.: Sampling effects, noise, and photobleaching in temporal image correlation spectroscopy. Biophys. J. 90, 628–639 (2006)
Kohlin, D.L., Wiseman, P.W.: Advances in image correlation spectroscopy: measuring number densities, aggregation states, and dynamics of fluorescently labeled macromolecules in cells. Cell Biochem. Biophys. 49, 141–164 (2007)
Keating, E., Nohe, A., Petersen, N.O.: Studies of distribution, location and dynamic properties of EGFR on the cell surface measured by image correlation spectroscopy. Eur. Biophys. J. 37, 469–481 (2008)
Spendier, K., Carroll-Portillo, A., Lidke, K.A., Wilson, B.S., Timlin, J.A., Thomas, J.L.: Distribution and dynamics of rat basophilic leukemia immunoglobulin E receptors (Fc\(\upvarepsilon \)RI) on planar ligand-presenting surfaces. Biophys. J. 99, 388–397 (2010)
Mohn, E., Stavem, P.: On the distribution of randomly placed discs. Biometrics 30, 137–156 (1974)
Abney, J.R., Scalettar, B.A., Hackenbrock, C.R.: On the measurement of particle number and mobility in nonideal solutions by fluorescence correlation spectroscopy. Biophys. J. 58, 261–265 (1990)
Wu, B., Chen, Y., Müller, J.D.: Fluorescence correlation spectroscopy of finite-sized particles. Biophys. J. 94, 2800–2808 (2008)
Kurniawan, N.A., Rajagopalan, R.: Probe-independent image correlation spectroscopy. Langmuir 27, 2775–2782 (2001)
Kruglov, T.: Correlation function of the excluded volume. J. Appl. Cryst. 38, 716–720 (2005)
Li, X., Shew, C.-Y., Liu, Y., Pynn, R., Liu, E., Herwig, K.W., Smith, G.S., Robertson, J.L., Chen, W.-R.: Prospect for characterizing interacting soft colloidal structures using spin-echo small angle neutron scattering. J. Chem. Phys. 134, 094504 (2011)
Stillinger, F.H.: Pair distribution in the classical rigid disk and sphere systems. J. Comp. Phys. 7, 367–384 (1971)
Guo, X., Riebel, U.: Theoretical direct correlation function for two-dimensional fluids of monodisperse hard spheres. J. Chem. Phys. 125, 144504 (2006)
Adda-Bedia, M., Katzav, E., Vella, D.: Solution of the Percus–Yevick equation for hard disks. J. Chem. Phys. 128, 184508 (2008)
Li, X., Shew, C.-Y., Liu, Y., Pynn, R., Liu, E., Herwig, K.W., Smith, G.S., Robertson, J.L., Chen, W.-R.: Theoretical studies on the structure of interacting colloidal suspensions by spin-echo small angle neutron scattering. J. Chem. Phys. 132, 174509 (2010)
Zhang, B., Zerubia, J., Olivo-Marin, J.-C.: Gaussian approximations of fluorescence microscope point-spread function models. Appl. Opt. 46, 1819–1829 (2007)
Attard, P., Bérard, D.R., Ursenbach, C.P., Patey, G.N.: Interaction free energy between planar walls in dense fluids: an Ornstein–Zernike approach with results for hard-sphere, Lennard–Jones, and dipolar systems. Phys. Rev. A 44, 8224–8234 (1991)
Holmes, T.J., Liu Y.: Richardson–Lucy/maximum likelihood image restoration algorithm for fluorescence microscopy: further testing. Appl. Opt. 28, 4930–4938 (1989)
Holmes, T.J.: Blind deconvolution of quantum limited incoherent imagery: maximum-likelihood approach. J. Opt. Soc. Am. A 9, 1052–1061 (1992)
Acknowledgements
K.S. was supported in part by the Program in Interdisciplinary Biological and Biomedical Sciences funded by the University of New Mexico and by Award Number T32EB009414 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Appendix: Hard ring image correlation spectroscopy
Appendix: Hard ring image correlation spectroscopy
The normalized intensity autocorrelation function of a ring of inner radius R 1 and outer radius R 2 can be derived from geometrical considerations. For \(2R_1 \leq \left( {R_{\,2} -R_1 } \right)\) the c.f. can be written as
and for \(\left( {R_{\,2} -R_1 } \right)\leq 2R_{\,2} \) one obtains
In Eqs. A.1 or A.2 g auto is given in Eq. 5 and from geometry the normalized intensity cross-correlation function g cross of two disks of radius R 1 and R 2 (\(R_{1} \ne R_{2})\) is
with
where \(h=R_{\,2} \sin \left( \theta \right)=R_1 \sin \left( \phi \right)\), \(\theta =\cos^{-1}\left( {\frac{R_{\,2}^{\,2}+r^{\,2}-R_1^2}{2R_{\,2} r}} \right)\), and \(\phi =\cos^{-1}\left( {\frac{R_1^{\,2}+r^{\,2}-R_{\,2}^{\,2}}{2R_1 r}} \right)\). Equations A.1 or A.2 can be substituted into Eq. 4 instead of the normalized intensity c.f. of a disk, Eq. 5, to obtain the rotationally averaged c.f. of randomly distributed hard rings with a uniform intensity profile, g ring(r), where the area fraction is defined as \(\eta =\rho \pi R_{\,2}^{\,2} \) in Eq. 8. To estimate the number of rings in a given observation volume one has to compute the appropriate scaling constant. Following Eq. 1 the scaling constant is given as
and the un-normalized rotationally averaged intensity c.f. for hard rings can be computed by evaluating
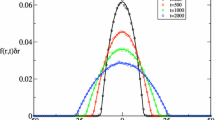
Equation A.6 was verified by simulating a test image (inset in Fig. 6a) of rings with inner radius R 1 = 5 pixels and outer radius R 2 = 10 pixels, w = 600 pixels, η = 0.30. These parameters were used to compute the theoretical intensity c.f. for hard rings. Figure 6a shows that the theoretical c.f. (solid line) fits the simulated data (open circles) quite well, verifying that the presented algorithm can also be applied to distributions of hard rings as expected. Following the presented algorithm one can obtain an expression for hard ring ICS by convolving Eqs. A.1 or A.2 with the autocorrelation of the Gaussian PSF:
Panel a shows the rotationally averaged spatial correlation function of a simulated distribution (inset) of 350 homogeneous hard rings (open circles) compared to the theoretical intensity correlation function given by Eq. A.6 (solid line) for R 1 = 5 pixels, R 2 = 10 pixels, w = 600 pixels, and η = 0.30. The theoretical intensity correlation function fits the simulated data quite well, validating the developed theory. Bar = 36 pixels. b Depicts the best fit (solid line) to the intensity correlation function (open circles) of a simulated microscope image (inset). Simulation parameters are the same as in a with σ = 6 pixels. Hard ring image correlation spectroscopy (ICS) given in Eq. A.7 fits the intensity c.f. of the blurred image very well even though one cannot distinguish between disks or rings by eye. Hard ring ICS estimates the true number of rings N, the inner radius R 1 as well as the outer radius R 2 within 10%, holding σ constant. Bar = 6 σ
C ′ is a scaling constant which can be estimated by fitting Eq. A.7 to the rotationally averaged intensity c.f. of a microscope image after deleting the g PSF(0) datum. Figure 6b shows the best fit (solid line) to the intensity c.f. (open circles) of a simulated microscope image (inset). Hard ring ICS fits the intensity c.f. of the blurred image very well even though one cannot distinguish between disks or rings by eye. Hard ring ICS estimates the true number of rings within 5%. The estimate of the inner radius R 1 is 0.78 σ and of the outer radius R 2 is 1.66 σ. Both values are within 10%.
Note that the correlation function of two infinitely narrow rings can be calculated exactly and gives a simple form. The correlation function for two infinitely narrow rings, each of radius R, is obtained by writing each ring as a delta function and calculating the overlap integral. With one ring centered at the origin and the other at r, the correlation is
where the prefactors are normalizations for the delta functions, and the law of cosines was used (θ ′ is the angle between the point of interest in the plane and the line between ring centers). The integral over r ′ is carried out by applying the first delta; to compute the integral over θ ′, the second delta is treated as a function of θ ′. Then
where the summation is taken over the zeroes of f , where θ \(^\prime = \theta_{0}^\prime \). When the rings overlap, there will be two zeroes of f , corresponding to the two points of overlap, and Eq. A.8 becomes
From geometry, \(\sin (\theta_0^\prime )=[1-r^2/(4R^{\,2})]^{1/2}\), completing the derivation.
Rights and permissions
About this article
Cite this article
Spendier, K., Thomas, J.L. Image correlation spectroscopy of randomly distributed disks. J Biol Phys 37, 477–492 (2011). https://doi.org/10.1007/s10867-011-9232-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-011-9232-x