Abstract
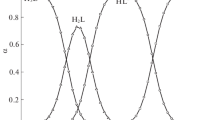
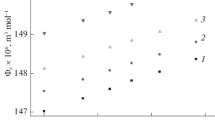
Interaction between aspartic acid and d-glucose, d-galactose, and d-fructose has been studied by isothermal titration calorimetry, calorimetry of dissolution, and densimetry. It has been found that d-glucose and d-fructose form thermodynamically stable associates with aspartic acid, in contrast to d-galactose. The selectivity in the interaction of aspartic acid with monosaccharides is affected by their stereochemical structures.
Similar content being viewed by others
References
Chang, L.C., Bewley, C.A.: Potent inhibition of HIV-1 fusion by cyanovirin-N requires a single high affinity carbohydrate binding site: characterization of low affinity carbohydrate binding site knockout mutants. J. Mol. Biol. 318, 1–8 (2002)
Westerlund, B., Korhonen, T.K.: Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9, 687–694 (1993)
Lemieux, R.U.: The origin of the specificity in the recognition of oligosaccharides by proteins. Chem. Soc. Rev. 18, 347–374 (1989)
Gorelic, E., Galili, U., Raz, A.: On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 20, 245–277 (2001)
Nidetzky, B., Eis, C., Albert, M.: Role of non-covalent enzyme-substrate interactions in the reaction catalysed by cellobiose phosphorylase from Cellulomonas uda. Biochem. J. 351, Pt.1. 649–659 (2000)
Toone, E.J.: Structure and energies of protein-carbohydrate complexes. Curr. Opin. Struct. Biol. 4, 719–728 (1994)
Weis, W.I., Drickamer, K.: Structural basis of lectin-carbohydrate recognition. Ann. Rev. Biochem. 65, 441–473 (1996)
Ambrosi, M., Cameron, N.R., Davis, B.G.: Lectins: tools for the molecular understanding of the glycocode. Organ. Biomol. Chem. 3, 1593–15608 (2005)
Cheng, Y., Shim, G., Kang, D., Kim, Y.: Carbohydrate binding specificity of pea lectin studied by NMR spectroscopy and molecular dynamics simulations. J. Mol. Struct. 475, 219–232 (1999)
Lebedeva, N.Sh., Mikhailovsky, K.V., Vyugin, A.I.: Differential calorimeter of titration. Russ. J. Phys. Chem. 75, 1140–1142 (2001)
Parfenyuk, E.V., Davydova, O.I., Lebedeva, N.Sh.: Interactions of d-maltose and sucrose with some amino acids in aqueous solutions. J. Solution Chem. 33, 1–10 (2004)
Volkova, N.L., Parfenyuk, E.V.: Selective interactions of 18-crown-6 with d-glucose and d-galactose in aqueous solutions: titration calorimetry, densimetry, viscosimetry. Thermochim. Acta. 435, 108–112 (2005)
Banipal, P.K., Banipal, T.S., Lark, B.S., Ahluwalia, J.C.: Partial molar heat capacities and volumes of some mono- di- and tri-saccharides in water at 298.15, 308.15 and 318.15 K. J. Chem. Soc. Faraday Trans. 93, 81–87 (1997)
Barone, G.: Physical chemistry of aqueous solutions of oligosaccharides. Thermochim. Acta. 162, 17–30 (1990)
Dey, P.C., Motin, M.A., Biswas, T.K., Huque, E.M.: Apparent molar volume and viscosity studies on some carbohydrates in solutions. Monatsh. Chem. 134, 797–809 (2003)
Schmidt, R.K., Karplus, M., Brady, J.M.: The anomeric equilibrium in d-xylose: free energy and the role of solvent structuring. J. Am. Chem. Soc. 118, 541–546 (1996)
Tvarovska, I.: Theoretical chemistry of biological systems (edited by G. Náray-Szabó), pp. 283–348. Elsevier, Amsterdam (1986)
Balk, R.W., Somsen, G.: Conformational aspects of the salvation of polyhydroxy compounds in binary mixtures of N,N-dimethylformamide and water. J. Solution Chem. 17, 139–152 (1988)
Aoyama, Y., Tanaka, Y., Sugahara, S.: Molecular recognition. 5. Molecular recognition of sugars via hydrogen-bonding interaction with a synthetic polyhydroxy macrocycle. J. Am. Chem. Soc. 111, 5397–5404 (1989)
Král, V., Rusin, O., Charvátová, J., Anzenbacher, P., Fogl, J.: Porphyrin phosphonates: novel anionic receptors for saccharide recognition. Tetrahedron Lett. 41, 10147–10151 (2000)
Volkova, N.L., Parfenyuk, E.V.: Parameters of the formation of molecular complexes in d-glucose–(d-galactose)–15-crown-5–water ternary solutions. Russ. J. Phys. Chem. A 81, 1151–1155 (2007)
Gabius, H.-J.: Biological information transfer beyond the genetic code: the sugar code. Naturwissenschaften 87, 108–121 (2000)
Parfenyuk, E.V., Davydova, O.V.: Stability constants for associates of saccharose and raffinose with glycine and dl-alanine in aqueous solutions. Russ. J. Phys. Chem. 78, 933–936 (2004)
Kulikova, G.A., Parfenyuk, E.V.: Influence of side chains of l-amino acids on their interaction with d-glucose in dilute aqueous solutions. J. Solut. Chem. (2008) (in press)
Gurney, R.W.: Ionic processes in solution. McGraw-Hill, New York (1954)
Mishra, A.K., Ahluwalia, J.C.: Apparent molal volumes of amino acids, N-acetylamino acids, and peptides in aqueous solutions. J. Phys. Chem. 88, 86–92 (1984)
Hedwig, G.R., Lilley, T.H., Linsdell, H.: Calorimetric and volumetric studies of the interactions of some amides in water and in 6 mol dm-3 aqueous guanidinium chloride. J. Chem. Soc. Faraday Trans. 87, 2975–2982 (1991)
Barone, G., Castronuovo, G., Del Vecchio, P., Elia, V., Tosto, M.T.: Thermodynamics of alcohols and monosaccharides in aqueous solutions of biuret at 25°C. J. Solution Chem. 17, 925–936 (1988)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulikova, G.A., Parfenyuk, E.V. Interaction Between Some Monosaccharides and Aspartic Acid in Dilute Aqueous Solutions. J Biol Phys 33, 247–254 (2007). https://doi.org/10.1007/s10867-008-9057-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-008-9057-4