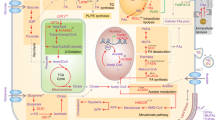
Abstract
Cancer cells can adapt their metabolic activity under nutritional hostile conditions in order to ensure both bioenergetics and biosynthetic requirements to survive. In this study, the effect of glucose deprivation on Caco-2 cells bioenergetics activity and putative relationship with membrane lipid changes were investigated. Glucose deprivation induces a metabolic remodeling characterized at mitochondrial level by an increase of oxygen consumption, arising from an improvement of complex II and complex IV activities and an inhibition of complex I activity. This effect is accompanied by changes in cellular membrane phospholipid profile. Caco-2 cells grown under glucose deprivation show higher phosphatidylethanolamine content and decreased phosphatidic acid content. Considering fatty acid profile of all cell phospholipids, glucose deprivation induces a decrease of monounsaturated fatty acid (MUFA) and n-3 polyunsaturated fatty acids (PUFA) simultaneously with an increase of n-6 PUFA, with consequent drop of n-3/n-6 ratio. Additionally, glucose deprivation affects significantly the fatty acid profile of all individual phospholipid classes, reflected by an increase of peroxidability index in zwitterionic phospholipids and a decrease in all anionic phospholipids, including mitochondrial cardiolipin. These data indicate that Caco-2 cells metabolic remodeling induced by glucose deprivation actively involves membrane lipid changes associated with a specific bioenergetics profile which ensure cell survival.
Similar content being viewed by others
References
Baritaki S, Apostolakis S, Kanellou P, Dimanche-Boitrel MT, Spandidos DA, Bonavida B (2007) Reversal of tumor resistance to apoptotic stimuli by alteration of membrane fluidity: therapeutic implications. Adv Cancer Res 98:149–190. doi:10.1016/S0065-230X(06)98005-1
Barrientos A, Fontanesi F, Diaz F (2009) Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr Protoc Hum Genet Chapter 19, Unit19 13. doi:10.1002/0471142905.hg1903s63
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234(3):466–468
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Campanella R (1992) Membrane lipids modifications in human gliomas of different degree of malignancy. J Neurosurg Sci 36(1):11–25
Canuto RA, Biocca ME, Muzio G, Dianzani MU (1989) Fatty acid composition of phospholipids in mitochondria and microsomes during diethylnitrosamine carcinogenesis in rat liver. Cell Biochem Funct 7(1):11–19. doi:10.1002/cbf.290070104
Chang NW, Wu CT, Chen DR, Yeh CY, Lin C (2013) High levels of arachidonic acid and peroxisome proliferator-activated receptor-alpha in breast cancer tissues are associated with promoting cancer cell proliferation. J Nutr Biochem 24(1):274–281. doi:10.1016/j.jnutbio.2012.06.005
Chicco AJ, Sparagna GC (2007) Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292(1):C33–C44. doi:10.1152/ajpcell.00243.2006
Das UN (2006) Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 1(4):420–439. doi:10.1002/biot.200600012
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104(49):19345–19350. doi:10.1073/pnas.0709747104
Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC et al (2005) Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol 25(22):10190–10201. doi:10.1128/MCB.25.22.10190-10201.2005
Fields RD, Lancaster MV (1993) Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am Biotechnol Lab 11(4):48–50
Filipp FV, Scott DA, Ronai ZA, Osterman AL, Smith JW (2012) Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res 25(3):375–383. doi:10.1111/j.1755-148X.2012.00989.x, Research Support, N.I.H., Extramural
Freeman MR, Solomon KR (2004) Cholesterol and prostate cancer. J Cell Biochem 91(1):54–69. doi:10.1002/jcb.10724
Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2(2):287–295. doi:10.1038/nprot.2006.478, Research Support, Non-U.S. Gov’t
Funahashi H, Satake M, Hasan S, Sawai H, Newman RA, Reber HA et al (2008) Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas 36(4):353–362. doi:10.1097/MPA.0b013e31815ccc44
Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ et al (2008) Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol 183(4):681–696. doi:10.1083/jcb.200803129
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Greiner EF, Guppy M, Brand K (1994) Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem 269(50):31484–31490, In Vitro Research Support, Non-U.S. Gov’t
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70, Research Support, U.S. Gov’t, Non-P.H.S.Research Support, U.S. Gov’t, P.H.S.Review
Hauff KD, Hatch GM (2006) Cardiolipin metabolism and Barth Syndrome. Prog Lipid Res 45(2):91–101. doi:10.1016/j.plipres.2005.12.001
Huang TC, Chen CP, Raftery A, Wefler V (1961) A stable reagent for Liebermann-Burchard reaction—application to rapid serum cholesterol determination. Anal Chem 33(10):1405–1407
Jose C, Melser S, Benard G, Rossignol R (2013) Mitoplasticity: adaptation biology of the mitochondrion to the cellular redox state in physiology and carcinogenesis. Antioxid Redox Signal 18(7):808–849. doi:10.1089/ars.2011.4357
Jowett M (1931) The phosphatide and cholesterol contents of normal and malignant human tissues. Biochem J 25(6):1991–1998
Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA et al (2009) Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med 46(11):1439–1453. doi:10.1016/j.freeradbiomed.2009.03.004
Kanthan R, Senger JL, Kanthan SC (2012) Molecular events in primary and metastatic colorectal carcinoma: a review. Pathol Res Int 2012:597497. doi:10.1155/2012/597497
Kolanjiappan K, Ramachandran CR, Manoharan S (2003) Biochemical changes in tumor tissues of oral cancer patients. Clin Biochem 36(1):61–65
Lepage G, Levy E, Ronco N, Smith L, Galeano N, Roy CC (1989) Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. J Lipid Res 30(10):1483–1490
Li YC, Park MJ, Ye SK, Kim CW, Kim YN (2006) Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol 168(4):1107–1118. doi:10.2353/ajpath.2006.050959, quiz 1404–1105
Madeira VM, Antunes-Madeira MC, Carvalho AP (1974) Activation energies of the ATPase activity of sarcoplasmic reticulum. Biochem Biophys Res Commun 58(4):897–904
Mashima T, Seimiya H, Tsuruo T (2009) De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer 100(9):1369–1372. doi:10.1038/sj.bjc.6605007, Research Support, Non-U.S. Gov’t Review
Mazurek J, Ignatowicz L, Kallenius G, Svenson SB, Pawlowski A, Hamasur B (2012) Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells. PLoS One 7(8):e42515. doi:10.1371/journal.pone.0042515
Melo T, Videira RA, Andre S, Maciel E, Francisco CS, Oliveira-Campos AM et al (2012) Tacrine and its analogues impair mitochondrial function and bioenergetics: a lipidomic analysis in rat brain. J Neurochem 120(6):998–1013. doi:10.1111/j.1471-4159.2011.07636.x
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7(10):763–777. doi:10.1038/nrc2222
Miura N, Matsumoto Y, Miyairi S, Nishiyama S, Naganuma A (1999) Protective effects of triterpene compounds against the cytotoxicity of cadmium in HepG2 cells. Mol Pharmacol 56(6):1324–1328
Munoz-Pinedo C, El Mjiyad N, Ricci JE (2012) Cancer metabolism: current perspectives and future directions. Cell Death Dis 3:e248. doi:10.1038/cddis.2011.123, Research Support, Non-U.S. Gov’tn Review
Murai T (2012) The role of lipid rafts in cancer cell adhesion and migration. Int J Cell Biol 2012:763283. doi:10.1155/2012/763283
Pegorier JP, Le May C, Girard J (2004) Control of gene expression by fatty acids. J Nutr 134(9):2444S–2449S, Research Support, Non-U.S. Gov’t Review
Peixoto F, Vicente J, Madeira VM (2004) A comparative study of plant and animal mitochondria exposed to paraquat reveals that hydrogen peroxide is not related to the observed toxicity. Toxicol in Vitro 18(6):733–739. doi:10.1016/j.tiv.2004.02.009
Peskin BS, Carter MJ (2008) Chronic cellular hypoxia as the prime cause of cancer: what is the de-oxygenating role of adulterated and improper ratios of polyunsaturated fatty acids when incorporated into cell membranes? Med Hypotheses 70(2):298–304. doi:10.1016/j.mehy.2007.05.033
Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Moreno-Sanchez R (2010) Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger. Mol Asp Med 31(1):29–59. doi:10.1016/j.mam.2009.12.006
Reitzer LJ, Wice BM, Kennell D (1979) Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem 254(8):2669–2676
Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA (2004) Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 64(3):985–993
Schlame M, Rua D, Greenberg ML (2000) The biosynthesis and functional role of cardiolipin. Prog Lipid Res 39(3):257–288
Schug ZT, Gottlieb E (2009) Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta 1788(10):2022–2031. doi:10.1016/j.bbamem.2009.05.004
Shelton LM, Huysentruyt LC, Seyfried TN (2010) Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer 127(10):2478–2485. doi:10.1002/ijc.25431
Shepherd D, Garland PB (1969) The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114(3):597–610, In Vitro
Smolkova K, Bellance N, Scandurra F, Genot E, Gnaiger E, Plecita-Hlavata L et al (2010) Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J Bioenerg Biomembr 42(1):55–67. doi:10.1007/s10863-009-9267-x
Stadlmann S, Renner K, Pollheimer J, Moser PL, Zeimet AG, Offner FA et al (2006) Preserved coupling of oxidative phosphorylation but decreased mitochondrial respiratory capacity in IL-1beta-treated human peritoneal mesothelial cells. Cell Biochem Biophys 44(2):179–186. doi:10.1385/CBB:44:2:179
Tawadros T, Brown MD, Hart CA, Clarke NW (2012) Ligand-independent activation of EphA2 by arachidonic acid induces metastasis-like behaviour in prostate cancer cells. Br J Cancer 107(10):1737–1744. doi:10.1038/bjc.2012.457
Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R et al (2010) Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18(3):207–219. doi:10.1016/j.ccr.2010.08.009
Warburg O (1956) On respiratory impairment in cancer cells. Science 124(3215):269–270
Weber K, Ridderskamp D, Alfert M, Hoyer S, Wiesner RJ (2002) Cultivation in glucose-deprived medium stimulates mitochondrial biogenesis and oxidative metabolism in HepG2 hepatoma cells. Biol Chem 383(2):283–290. doi:10.1515/BC.2002.030
Weinberg F, Chandel NS (2009) Mitochondrial metabolism and cancer. Ann N Y Acad Sci 1177:66–73. doi:10.1111/j.1749-6632.2009.05039.x
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M et al (2010) Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 107(19):8788–8793. doi:10.1073/pnas.1003428107
Yamaguchi H, Oikawa T (2010) Membrane lipids in invadopodia and podosomes: key structures for cancer invasion and metastasis. Oncotarget 1(5):320–328, Research Support, Non-U.S. Gov’t Review
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 4.92 MB)
Rights and permissions
About this article
Cite this article
Monteiro-Cardoso, V.F., Silva, A.M., Oliveira, M.M. et al. Membrane lipid profile alterations are associated with the metabolic adaptation of the Caco-2 cells to aglycemic nutritional condition. J Bioenerg Biomembr 46, 45–57 (2014). https://doi.org/10.1007/s10863-013-9531-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-013-9531-y