Abstract
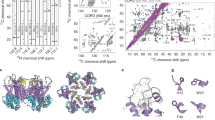
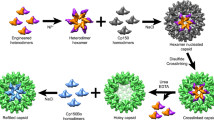
Recent studies of noncrystalline HIV-1 capsid protein (CA) assemblies by our laboratory and by Polenova and coworkers (Protein Sci 19:716–730, 2010; J Mol Biol 426:1109–1127, 2014; J Biol Chem 291:13098–13112, 2016; J Am Chem Soc 138:8538–8546, 2016; J Am Chem Soc 138:12029–12032, 2016; J Am Chem Soc 134:6455–6466, 2012; J Am Chem Soc 132:1976–1987, 2010; J Am Chem Soc 135:17793–17803, 2013; Proc Natl Acad Sci USA 112:14617–14622, 2015; J Am Chem Soc 138:14066–14075, 2016) have established the capability of solid state nuclear magnetic resonance (NMR) measurements to provide site-specific structural and dynamical information that is not available from other types of measurements. Nonetheless, the relatively high molecular weight of HIV-1 CA leads to congestion of solid state NMR spectra of fully isotopically labeled assemblies that has been an impediment to further progress. Here we describe an efficient protocol for production of segmentally labeled HIV-1 CA samples in which either the N-terminal domain (NTD) or the C-terminal domain (CTD) is uniformly 15N,13C-labeled. Segmental labeling is achieved by trans-splicing, using the DnaE split intein. Comparisons of two-dimensional solid state NMR spectra of fully labeled and segmentally labeled tubular CA assemblies show substantial improvements in spectral resolution. The molecular structure of HIV-1 assemblies is not significantly perturbed by the single Ser-to-Cys substitution that we introduce between NTD and CTD segments, as required for trans-splicing.






Similar content being viewed by others
References
Antos JM, Truttmann MC, Ploegh HL (2016) Recent advances in sortase-catalyzed ligation methodology. Curr Opin Struct Biol 38:111–118
Balambika R, Inui T, Sargsyan H, Arshava B, Cohen LS, Ding FX, Becker JM, Naider F (2007) Synthesis of a double transmembrane domain fragment of Ste2p by native chemical ligation. Int J Pept Res Ther 13:251–263
Barklis E, Alfadhli A, McQuaw C, Yalamuri S, Still A, Barklis RL, Kukull B, Lopez CS (2009) Characterization of the in vitro HIV-1 capsid assembly pathway. J Mol Biol. 387:376–389
Bayro MJ, Tycko R (2016) Structure of the dimerization interface in the mature HIV-1 capsid protein lattice from solid state NMR of tubular assemblies. J Am Chem Soc 138:8538–8546
Bayro MJ, Chen B, Yau WM, Tycko R (2014) Site-specific structural variations accompanying tubular assembly of the HIV-1 capsid protein. J Mol Biol 426:1109–1127
Bayro MJ, Ganser-Pornillos BK, Zadrozny KK, Yeager M, Tycko R (2016) Helical conformation in the CA-SP1 junction of the immature HIV-1 lattice determined from solid state NMR of virus-like particles. J Am Chem Soc 138:12029–12032
Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG (1995) Heteronuclear decoupling in rotating solids. J Chem Phys 103:6951–6958
Briggs JAG, Kräusslich HG (2011) The molecular architecture of HIV. J Mol Biol 410:491–500
Byeon IJL, Meng X, Jung J, Zhao G, Yang R, Ahn J, Shi J, Concel J, Aiken C, Zhang P, Gronenborn AM (2009) Structural convergence between cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function. Cell 139:780–790
Byeon IJL, Hou G, Han Y, Suiter CL, Ahn J, Jung J, Byeon CH, Gronenborn AM, Polenova T (2012) Motions on the millisecond time scale and multiple conformations of HIV-1 capsid protein: implications for structural polymorphism of CA assemblies. J Am Chem Soc 134:6455–6466
Camarero JA, Shekhtman A, Campbell EA, Chlenov M, Gruber TM, Bryant DA, Darst SA, Cowburn D, Muir TW (2002) Autoregulation of a bacterial σ factor explored by using segmental isotopic labeling and NMR. Proc Natl Acad Sci USA 99:8536–8541
Chen B, Tycko R (2010) Structural and dynamical characterization of tubular HIV-1 capsid protein assemblies by solid state nuclear magnetic resonance and electron microscopy. Protein Sci 19:716–730
David R, Richter MPO, Beck-Sickinger AG (2004) Expressed protein ligation: method and applications. Eur J Biochem 271:663–677
Dawson PE, Kent SBH (2000) Synthesis of native proteins by chemical ligation. Annu Rev Biochem 69:923–960
Dawson PE, Muir TW, Clark-Lewis I, Kent SBH (1994) Synthesis of proteins by native chemical ligation. Science 266:776–779
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A, NMRpipe (1995) A multidimensional spectral processing system based on Unix pipes. J Biomol NMR 6:277–293
Deshmukh L, Schwieters CD, Grishaev A, Ghirlando R, Baber JL, Clore GM (2013) Structure and dynamics of full-length HIV-1 capsid protein in solution. J Am Chem Soc 135:16133–16147
Engelman A, Cherepanov P (2012) The structural biology of HIV-1: mechanistic and therapeutic insights. Nature Rev Microbiol 10:279–290
Frederick KK, Michaelis VK, Caporini MA, Andreas LB, Debelouchina GT, Griffin RG, Lindquist S (2017) Combining DNP NMR with segmental and specific labeling to study a yeast prion protein strain that is not parallel in-register. Proc Natl Acad Sci USA 114:3642–3647
Freiburger L, Sonntag M, Hennig J, Li J, Zou P, Sattler M (2015) Efficient segmental isotope labeling of multi-domain proteins using sortase A. J Biomol NMR 63:1–8
Gamble TR, Yoo SH, Vajdos FF, vonSchwedler UK, Worthylake DK, Wang H, McCutcheon JP, Sundquist WI, Hill CP (1997) Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278:849–853
Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI (1999) Assembly and analysis of conical models for the HIV-1 core. Science 283:80–83
Ganser-Pornillos BK, Cheng A, Yeager M (2007) Structure of full-length HIV-1 CA: a model for the mature capsid lattice. Cell 131:70–79
Ganser-Pornillos BK, Yeager M, Sundquist WI (2008) The structural biology of HIV assembly. Curr Opin Struct Biol 18:203–217
Gitti RK, Lee BM, Walker J, Summers MF, Yoo S, Sundquist WI (1996) Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science 273:231–235
Gres AT, Kirby KA, KewalRamani VN, Tanner JJ, Pornillos O, Sarafianos SG (2015) X-ray crystal structures of native HIV-1 capsid protein reveal conformational variability. Science 349:99–103
Han Y, Ahn J, Concel J, Byeon IJL, Gronenborn AM, Yang J, Polenova T (2010) Solid state NMR studies of HIV-1 capsid protein assemblies. J Am Chem Soc 132:1976–1987
Han Y, Hou G, Suiter CL, Ahn J, Byeon IJL, Lipton AS, Burton S, Hung I, Gorkov PL, Gan Z, Brey W, Rice D, Gronenborn AM, Polenova T (2013) Magic angle spinning NMR reveals sequence-dependent structural plasticity, dynamics, and the spacer peptide 1 conformation in HIV-1 capsid protein assemblies. J Am Chem Soc 135:17793–17803
Iwai H, Züger S, Jin J, Tam PH (2006) Highly efficient protein trans-splicing by a naturally split DnaE intein from Nostoc punctiforme. FEBS Lett 580:1853–1858
Jackson DY, Burnier J, Quan C, Stanley M, Tom J, Wells JA (1994) A designed peptide ligase for total synthesis of ribonuclease A with unnatural catalytic residues. Science 266:243–247
Jiang J, Ablan SD, Derebail S, Hercík K, Soheilian F, Thomas JA, Tang S, Hewlett I, Nagashima K, Gorelick RJ, Freed EO, Levin JG (2011) The interdomain linker region of HIV-1 capsid protein is a critical determinant of proper core assembly and stability. Virology 421:253–265
Kobashigawa Y, Kumeta H, Ogura K, Inagaki F (2009) Attachment of an NMR-invisible solubility enhancement tag using a sortase-mediated protein ligation method. J Biomol NMR 43:145–150
Kochendoerfer GG, Jones DH, Lee S, Oblatt-Montal M, Opella SJ, Montal M (2004) Functional characterization and NMR spectroscopy on full-length Vpu from HIV-1 prepared by total chemical synthesis. J Am Chem Soc 126:2439–2446
Kwon B, Tietze D, White PB, Liao SY, Hong M (2015) Chemical ligation of the influenza M2 protein for solid state NMR characterization of the cytoplasmic domain. Protein Sci 24:1087–1099
Li Y (2015) Split-inteins and their bioapplications. Biotechnol Lett 37:2121–2137
Li S, Hill CP, Sundquist WI, Finch JT (2000) Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature 407:409–413
Liu DS, Cowburn D (2017) Segmental isotopic labeling of proteins for NMR study using intein technology. In: Mootz HD (ed) Split inteins: methods and protocols, vol 1495. Springer, New York, pp 131–145
Liu DS, Yuan Y, Xu R, Cowburn D (2017) Domain interactions of C-terminal Src kinase determined through NMR spectroscopy with segmental isotope labeling. Protein Cell 8:67–71
Lockless SW, Muir TW (2009) Traceless protein splicing utilizing evolved split inteins. Proc Natl Acad Sci USA 106:10999–11004
Lu M, Hou G, Zhang H, Suiter CL, Ahn J, Byeon IJL, Perilla JR, Langmead CJ, Hung I, Gor’kov PL, Gan Z, Brey W, Aiken C, Zhang P, Schulten K, Gronenborn AM, Polenova T (2015) Dynamic allostery governs cyclophilin A-HIV capsid interplay. Proc Natl Acad Sci USA 112:14617–14622
Lu JX, Bayro MJ, Tycko R (2016) Major variations in HIV-1 capsid assembly morphologies involve minor variations in molecular structures of structurally ordered protein segments. J Biol Chem 291:13098–13112
Marulanda D, Tasayco ML, McDermott A, Cataldi M, Arriaran V, Polenova T (2004) Magic angle spinning solid state NMR spectroscopy for structural studies of protein interfaces. Resonance assignments of differentially enriched Escherichia coli thioredoxin reassembled by fragment complementation. J Am Chem Soc 126:16608–16620
Mattei S, Glass B, Hagen WJH, Kräusslich HG, Briggs JAG (2016) The structure and flexibility of conical HIV-1 capsids determined within intact virions. Science 354:1434–1437
McNeill SA, Gor’kov PL, Shetty K, Brey WW, Long JR (2009) A low-E magic angle spinning probe for biological solid state NMR at 750 MHz. J Magn Reson 197:135–144
Mehler M, Eckert CE, Busche A, Kulhei J, Michaelis J, Becker-Baldus J, Wachtveitl J, Dötsch V, Glaubitz C (2015) Assembling a correctly folded and functional heptahelical membrane protein by protein trans-splicing. J Biol Chem 290:27712–27722
Michel E, Skrisovska L, Wüthrich K, Allain FHT (2013) Amino acid-selective segmental isotope labeling of multidomain proteins for structural biology. ChemBioChem 14:457–466
Minato Y, Ueda T, MacHiyama A, Shimada I, Iwaï H (2012) Segmental isotopic labeling of a 140 kDa dimeric multi-domain protein chea from Escherichia coli by expressed protein ligation and protein trans-splicing. J Biomol NMR 53:191–207
Morcombe CR, Gaponenko V, Byrd RA, Zilm KW (2004) Diluting abundant spins by isotope edited radio frequency field assisted diffusion. J Am Chem Soc 126:7196–7197
Muona M, Aranko AS, Iwai H (2008) Segmental isotopic labelling of a multidomain protein by protein ligation by protein trans-splicing. ChemBioChem 9:2958–2961
Muralidharan V, Muir TW (2006) Protein ligation: an enabling technology for the biophysical analysis of proteins. Nat Methods 3:429–438
Murray DT, Kato M, Lin Y, Thurber KR, Hung I, McKnight SL, Tycko R (2017) Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell. https://doi.org/10.1016/j.cell.2017.08.048
Nguyen GKT, Cao Y, Wang W, Liu CF, Tam JP (2015) Site-specific N-terminal labeling of peptides and proteins using butelase 1 and thiodepsipeptide. Angew Chem Int Ed 54:15694–15698
Pornillos O, Ganser-Pornillos BK, Kelly BN, Hua Y, Whitby FG, Stout CD, Sundquist WI, Hill CP, Yeager M (2009) X-ray structures of the hexameric building block of the HIV capsid. Cell 137:1282–1292
Pornillos O, Ganser-Pornillos BK, Yeager M (2011) Atomic-level modelling of the HIV capsid. Nature 469:424–427
Rajagopal S, Kent SBH (2007) Total chemical synthesis and biophysical characterization of the minimal isoform of the KChIP2 potassium channel regulatory subunit. Protein Sci 16:2056–2064
Reuther G, Tan KT, Vogel A, Nowak C, Arnold K, Kuhlmann J, Waldmann H, Huster D (2006) The lipidated membrane anchor of full length N-Ras protein shows an extensive dynamics as revealed by solid state NMR spectroscopy. J Am Chem Soc 128:13840–13846
Rihn SJ, Wilson SJ, Loman NJ, Alim M, Bakker SE, Bhella D, Gifford RJ, Rixon FJ, Bieniasz PD (2013) Extreme genetic fragility of the HIV-1 capsid. PLoS Path 9:e1003461
Schubeis T, Lührs T, Ritter C (2015a) Unambiguous assignment of short- and long-range structural restraints by solid state NMR spectroscopy with segmental isotope labeling. ChemBioChem 16:51–54
Schubeis T, Yuan P, Ahmed M, Nagaraj M, Van Rossum BJ, Ritter C (2015b) Untangling a repetitive amyloid sequence: correlating biofilm-derived and segmentally labeled curli fimbriae by solid state NMR spectroscopy. Angew Chem Int Ed 54:14669–14672
Schur FKM, Obr M, Hagen WJH, Wan W, Jakobi AJ, Kirkpatrick JM, Sachse C, Krausslich HG, Briggs JA (2016) An atomic model of HIV-1 capsid-SP1 reveals structures regulating assembly and maturation. Science 353:506–508
Severinov K, Muir TW (1998) Expressed protein ligation, a novel method for studying protein-protein interactions in transcription. J Biol Chem 273:16205–16209
Shah NH, Muir TW (2014) Inteins: nature’s gift to protein chemists. Chem Sci 5:446–461
Shin R, Tzou YM, Krishna NR (2011) Structure of a monomeric mutant of the HIV-1 capsid protein. Biochemistry 50:9457–9467
Skrisovska L, Allain FHT (2008) Improved segmental isotope labeling methods for the NMR study of multidomain or large proteins: application to the RRMs of NpI3p and hnRNP L. J Mol Biol 375:151–164
Stevens AJ, Brown ZZ, Shah NH, Sekar G, Cowburn D, Muir TW (2016) Design of a split intein with exceptional protein splicing activity. J Am Chem Soc 138:2162–2165
Takegoshi K, Nakamura S, Terao T (2001) 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett 344:631–637
Vitali F, Henning A, Oberstrass FC, Hargous Y, Auweter SD, Erat M, Allain FH (2006) Structure of the two most C-terminal RNA recognition motifs of PTB using segmental isotope labeling. EMBO J 25:150–162
Volkmann G, Iwaï H (2010) Protein trans-splicing and its use in structural biology: opportunities and limitations. Mol BioSys 6:2110–2121
Wagner JM, Zadrozny KK, Chrustowicz J, Purdy MD, Yeager M, Ganser-Pornillos BK, Pornillos O (2016) Crystal structure of an HIV assembly and maturation switch. eLife 5:e17063
Williams FP, Milbradt AG, Embrey KJ, Bobby R (2016) Segmental isotope labelling of an individual bromodomain of a tandem domain BRD4 using sortase A. PLoS ONE 11:e0154607
Wong HC, Shin R, Krishna NR (2008) Solution structure of a double mutant of the carboxy-terminal dimerization domain of the HIV-1 capsid protein. Biochemistry 47:2289–2297
Xu R, Ayers B, Cowburn D, Muir TW (1999) Chemical ligation of folded recombinant proteins: segmental isotopic labeling of domains for NMR studies. Proc Natl Acad Sci USA 96:388–393
Yagi H, Tsujimoto T, Yamazaki T, Yoshida M, Akutsu H (2004) Conformational change of H+-ATPase β monomer revealed on segmental isotope labeling NMR spectroscopy. J Am Chem Soc 126:16632–16638
Zettler J, Schütz V, Mootz HD (2009) The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans-splicing reaction. FEBS Lett 583:909–914
Zhang Y, Vasudevan S, Sojitrawala R, Zhao W, Cui C, Xu C, Fan D, Newhouse Y, Balestra R, Jerome WG, Weisgraber K, Li Q, Wang J (2007) A monomeric, biologically active, full-length human apolipoprotein E. Biochemistry 46:10722–10732
Zhang H, Hou G, Lu M, Ahn J, Byeon IJL, Langmead CJ, Perilla JR, Hung I, Gor’Kov PL, Gan Z, Brey WW, Case DA, Schulten K, Gronenborn AM, Polenova T (2016) HIV-1 capsid function is regulated by dynamics: quantitative atomic-resolution insights by integrating magic-angle-spinning NMR, QM/MM, and MD. J Am Chem Soc 138:14066–14075
Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, Ahn J, Gronenborn AM, Schulten K, Aiken C, Zhang P (2013) Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 497:643–646
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and the Intramural AIDS Targeted Antiviral Program of the National Institutes of Health. We thank Dr. Marvin J. Bayro for generous assistance and helpful advice regarding HIV-1 CA assembly conditions, electron microscopy, and solid state NMR spectroscopy. We thank John R. Lloyd for performing mass spectrometry of our ligated products.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, S., Tycko, R. Segmental isotopic labeling of HIV-1 capsid protein assemblies for solid state NMR. J Biomol NMR 70, 103–114 (2018). https://doi.org/10.1007/s10858-017-0162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-017-0162-1