Abstract
A solid state NMR experiment is introduced for probing relatively slow conformational exchange, based on dephasing and refocusing dipolar couplings. The method is closely related to the previously described Centerband-Only Detection of Exchange or CODEX experiment. The use of dipolar couplings for this application is advantageous because their values are known a priori from molecular structures, and their orientations and reorientations relate in a simple way to molecular geometry and motion. Furthermore the use of dipolar couplings in conjunction with selective isotopic enrichment schemes is consistent with selection for unique sites in complex biopolymers. We used this experiment to probe the correlation time for the motion of 13C, 15N enriched urea molecules within their crystalline lattice.





Similar content being viewed by others
References
Benkovic SJ, Hammes-Schiffer S (2003) A perspective on enzyme catalysis. Science 301:1196–1202
Chiba T (1965) A deuteron magnetic resonance study of urea-d4. Bull Chem Soc Jpn 38:259–263
DeAzevedo ER, Hu W-G, Bonagamba TJ, Schmidt-Rohr K (1999) Centerband-only detection of exchange: efficient analysis of dynamics in solids by NMR. J Am Chem Soc 121:8411–8412
DeAzevedo ER, Hu W-G, Bonagamba TJ, Schmidt-Rohr K (2000) Principles of centerband-only detection of exchange in solid-state nuclear magnetic resonance, and extension to four-time centerband-only detection of exchange. J Chem Phys 112:8988–9001
Detken A, Hardy EH, Ernst M, Kainosho M, Kawakami T, Aimoto S, Meier BH (2001) Methods for sequential resonance assignment in solid, uniformly 13C, 15N labeled peptides: quantification and application to antamanide. J Biomol NMR 20:8411–8412
Emsley JW, Smith JAS (1961) Proton magnetic resonance studies of amides. Part 2: molecular motion in thiourea and urea. Trans Faraday Soc 57:1233–1247
Gullion T, Schaefer J (1989) Rotational-echo double-resonance NMR. J Magn Reson 81:196–200
Havlin R, Le H, Laws D, deDios A, Oldfield E (1997) An ab initio quantum chemical investigation of carbon-13 NMR shielding tensors in glycine, alanine, valine, isoleucine, serine, and threonine: comparisons between helical and sheet tensors, and the effects of on shielding. J Am Chem Soc 119:11951–11958
Noji H, Yasuda R, Yoshida M, Kinosita K Jr (1997) Direct observation of the rotation of F1-ATPase. Nature 386:299–302
Reichert D, Pascui O (2003) Scaling-down the CSA recoupling in S-CODEX 1D-MAS exchange experiments. Chem Phys Lett 380:583–588
Reichert D, Pascui O (2008) CONTRA: improving the performance of dynamic investigations in natural abundance organic solids by mirror-symmetric constant-time CODEX. J Magn Reson 191:141–147
Sitkoff D, Case D (1998) Theories of chemical shift anisotropies in proteins and nucleic acids. Prog Nucl Magn Reson Spectrosc 32:165–190
Sørensen OW, Eich GW, Levitt MH, Bodenhausen G, Ernst RR (1983) Product operator formalism for the description of NMR pulse experiments. Progr NMR Spextrosc 16:163–192
Taylor RE, Bacher AD, Dybowski C (2007) 1H NMR relaxation in urea. J Mol Struct 846:147–152
Vale RD, Milligan RA (2000) The way things move: looking under the hood of molecular motor proteins. Science 288:88–95
Vaughan P, Donohue J (1952) The structure of urea. Interatomic distances and resonance in urea and related compounds. Acta Cryst 5:530–535
Veshtort M, Griffin RG (2006) SPINEVOLUTION: a powerful tool for the simulation of solid and liquid state NMR experiments. J Magn Reson 178:248–282
Williams JC, McDermott AE (1993) Cis-trans energetics in urea and acetamide studied by deuterium NMR. J Phys Chem 97:12393–12398
Zussman A (1973) Effect of molecular reorientation in urea on the 14N PNQR linewidth and relaxation time. J Chem Phys 58:1514–1522
Acknowledgments
We thank Dr. Yisong Tao for experimental assistance.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
First, we will describe the recoupling of the heteronuclear dipolar coupling under MAS condition. The average Hamiltonian dominant in the evolution during an interval (t 0, t) is
where ϕ(t 0, t) is the phase developed by the dipolar coupling during the interval,
The laboratory frame tensor \( \Uplambda_{20}^{\text{Lab}} (\omega_{R} t) \) can be obtained by transforming the dipolar coupling’s uniaxial tensor from the principal axis frame to the rotor frame through the Euler angles {α, β, γ}, and then to the laboratory frame, which causes a time-dependent due to magic angle spinning. For the REDOR pulse sequence element used during the dephasing and refocusing parts, π pulses invert the sign of the dipolar coupling Hamiltonian for every other half rotor period. Therefore, the phase accumulated in one rotor period (Tr) is
where t 0 defines the initial rotor phase ω R t 0. Therefore, after the N rotor periods during the dephasing or refocusing time, the total phase which the magnetization evolves is
which shows that the phase is scaled by a sine function of the Euler angle β. Thus the two exchange sites, which have different Euler angles {α, β, γ} transforming their dipolar coupling tensor from principal to rotor frame, will develop different phases under the dominant of the dipolar coupling Hamiltonian. In the following, the product operator formalism (Sørensen et al. 1983) and an exchange matrix description are applied to illustrate how this phase difference can detect the dynamics of the two exchange sites.
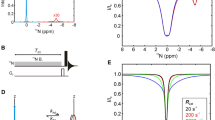
The dynamical model we used is a two-site exchange model for a single 13C–15N pair (Fig. 5b), and we assume that there is no exchange during the dephasing and refocusing parts (although for numerical simulations the exchange during those periods was included). After cross polarization between proton and carbon, the resulting × magnetizations of the two exchange sites evolve under the action of 13C–15N recoupled heteronuclear dipolar coupling during the dephasing time.
Then a 90° pulse (phase cycle is indicated in Table 2) on 13C flips the antiphase magnetization to generate longitudinal two spin order. (The remaining in-phase part will disappear during mixing time due to spin-spin relaxation and is therefore omitted.) Then, in the mixing time, the exchange process redistributes the magnetization between two exchange sites. After the exchange process,
where t m is the length of the mixing time, k ex is the exchange rate which is the summation of the forward and backward rates.
Finally, the 90 pulse on carbon flips the carbon’s part back to the y direction and the refocusing part makes the antiphase part evolve back to x direction,
Therefore, the final signal should be
The difference between urea model (Fig. 5a) and the single 13C–15N model (Fig. 5b) is that two nitrogen atoms connected with the center carbon atom simultaneously in urea. Therefore, after dephasing time, the carbon’s magnetization in urea is
where φ1 and φ2 are the phases developed under the two 13C–15N dipolar couplings separately. The terms in the first bracket of Eq. (11) are similar to the “SinSin” portion in original CODEX publication (DeAzevedo et al. 2000, 90 x or 90−x pulse can flip them to the z-direction) while those terms in the second bracket are similar to “CosCos” portion (90 y or 90−y can flip them to the z-direction). The reorientation process which exchanges N1 and N2 only affects the “SinSin” portion. Therefore, only “SinSin” portion was obtained in our experiment. The final signal of “SinSin” portion is
Equation (12) is a little different from Eq. (10) but the signal in this equation has the same mixing time dependence with that in Eq. (10).
Rights and permissions
About this article
Cite this article
Li, W., McDermott, A.E. Characterization of slow conformational dynamics in solids: dipolar CODEX. J Biomol NMR 45, 227–232 (2009). https://doi.org/10.1007/s10858-009-9353-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-009-9353-8