Abstract
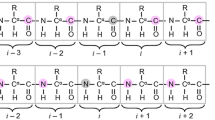
Triple resonance E.COSY-based techniques were used to measure intra-residue and sequential HN–Hα residual dipolar couplings (RDCs) for the third IgG-binding domain of protein G (GB3), aligned in Pf1 medium. Measurements closely correlate with values predicted on the basis of an NMR structure, previously determined on the basis of a large number of one-bond backbone RDCs measured in five alignment media. However, in particular the sequential HN–Hα RDCs are smaller than predicted for a static structure, suggesting a degree of motion for these internuclear vectors that exceeds that of the backbone amide N–H vectors. Of all experimentally determined GB3 structures available, the best correlation between experimental 1H–1H couplings is observed for a GB3 ensemble, previously derived to generate a realistic picture of the conformational space sampled by GB3 (Clore and Schwieters, J Mol Biol 355:879–886, 2006). However, for both NMR and X-ray-derived structures the 1H–1H couplings are found to be systematically smaller than expected on the basis of alignment tensors derived from 15N–1H amide RDCs, assuming librationally corrected N–H bond lengths of 1.041 Å.






Similar content being viewed by others
References
Bax A, Kontaxis G, Tjandra N (2001) Dipolar couplings in macromolecular structure determination. Meth Enzymol 339:127–174
Biamonti C, Rios CB, Lyons BA, Montelione GT (1994) Multidimensional NMR experiments and analysis techniques for determining homo- and heteronuclear scalar coupling constants in proteins and nucleic acids. Adv Biophys Chem 4:51–120
Bolon PJ, Prestegard JH (1998) COSY cross-peaks from H-1–H-1 dipolar couplings in NMR spectra of field oriented oligosaccharides. J Am Chem Soc 120:9366–9367
Bouvignies G, Bernado P, Meier S, Cho K, Grzesiek S, Bruschweiler R, Blackledge M (2005) Identification of slow correlated motions in proteins using residual dipolar and hydrogen-bond scalar couplings. Proc Natl Acad Sci USA 102:13885–13890
Cai ML, Wang H, Olejniczak ET, Meadows RP, Gunasekera AH, Xu N, Fesik SW (1999) Accurate measurement of H–N–H-alpha residual dipolar couplings in proteins. J Magn Reson 139:451–453
Case DA (1999) Calculations of NMR dipolar coupling strengths in model peptides. J Biomol NMR 15:95–102
Clore GM, Schwieters CD (2006) Concordance of residual dipolar couplings, backbone order parameters and crystallographic B-factors for a small alpha/beta protein: a unified picture of high probability, fast atomic motions in proteins. J Mol Biol 355:879–886
Clore GM, Gronenborn AM, Bax A (1998a) A robust method for determining the magnitude of the fully asymmetric alignment tensor of oriented macromolecules in the absence of structural information. J Magn Reson 133:216–221
Clore GM, Starich MR, Gronenborn AM (1998b) Measurement of residual dipolar couplings of marcomolecules aligned in the nematic phase of a colloidal suspension of rod-shaped viruses. J Am Chem Soc 120:10571–10572
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRpipe—a multidimensional spectral processing system based on Unix pipes. J Biomol NMR 6:277–293
Derrick JP, Wigley DB (1994) The 3rd IgG-binding domain from streptococcal protein-G—an analysis by X-ray crystallography of the structure alone and in a complex with fab. J Mol Biol 243:906–918
Eisenmesser EZ, Millet O, Labeikovsky W, Korzhnev DM, Wolf-Watz M, Bosco DA, Skalicky JJ, Kay LE, Kern D (2005) Intrinsic dynamics of an enzyme underlies catalysis. Nature 438:117–121
Engh RA, Huber R (1991) Accurate bond and angle parameters for X-ray protein-structure refinement. Acta Crystallographica Section A 47:392–400
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141
Griesinger C, Sorensen OW, Ernst RR (1985) Two-dimensional correlation of connected NMR transitions. J Am Chem Soc 107:6394–6396
Griesinger C, Sørensen OW, Ernst RR (1986) Correlation of connected transitions by two-dimensional NMR spectroscopy. J Chem Phys 85:6837–6852
Hall JB, Fushman D (2003) Characterization of the overall and local dynamics of a protein with intermediate rotational anisotropy: differentiating between conformational exchange and anisotropic diffusion in the B3 domain of protein G. J Biomol NMR 27:261–275
Hansen MR, Mueller L, Pardi A (1998a) Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions. Nat Struct Biol 5:1065–1074
Hansen MR, Rance M, Pardi A (1998b) Observation of long-range H-1–H-1 distances in solution by dipolar coupling interactions. J Am Chem Soc 120:11210–11211
Harbison GS (1993) Interference between J-couplings and cross-relaxation in solution NMR-spectroscopy—consequences for macromolecular structure determination. J Am Chem Soc 115:3026–3027
Hu KF, Vogeli B, Clore GM (2006) Interference between transverse cross-correlated relaxation and longitudinal relaxation affects apparent J-coupling and transverse cross-correlated relaxation. Chem Phys Lett 423:123–125
Ishima R, Torchia DA (2000) Protein dynamics from NMR. Nat Struct Biol 7:740–743
Koradi R, Billeter M, Wuthrich K (1996) MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph 14:51–55
Lindorff-Larsen K, Best RB, DePristo MA, Dobson CM, Vendruscolo M (2005) Simultaneous determination of protein structure and dynamics. Nature 433:128–132
Lipari G, Szabo A (1982) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc 104:4546–4559
Losonczi JA, Andrec M, Fischer MWF, Prestegard JH (1999) Order matrix analysis of residual dipolar couplings using singular value decomposition. J Magn Reson 138:334–342
Meiler J, Prompers JJ, Peti W, Griesinger C, Bruschweiler R (2001) Model-free approach to the dynamic interpretation of residual dipolar couplings in globular proteins. J Am Chem Soc 123:6098–6107
Montelione GT, Wagner G (1989) Accurate measurements of homonuclear H–N–H-alpha coupling-constants in polypeptides using heteronuclear 2D NMR experiments. J Am Chem Soc 111:5474–5475
Mulder FAA, Mittermaier A, Hon B, Dahlquist FW, Kay LE (2001) Studying excited states of proteins by NMR spectroscopy. Nat Struct Biol 8:932–935
Ottiger M, Bax A (1998) Determination of relative N–H–N N–C′, C-alpha-C′, and C(alpha)–H-alpha effective bond lengths in a protein by NMR in a dilute liquid crystalline phase. J Am ChemSoc 120:12334–12341
Palmer AG, Kroenke CD, Loria JP (2001). Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Nucl Magn Reson Biol Macromol Pt B 339:204–238
Pellecchia M, Vander Kooi CW, Keliikuli K, Zuiderweg ERP (2000) Magnetization transfer via residual dipolar couplings: application to proton–proton correlations in partially aligned proteins. J Magn Reson 143:435–439
Peng JW, Wagner G (1992) Mapping of spectral density functions using heteronuclear NMR relaxation measurements. J Magn Reson 98:308–332
Peti W, Griesinger C (2000) Measurement of magnitude and sign of H, H-dipolar couplings in proteins. J Am Chem Soc 122:3975–3976
Peti W, Meiler J, Bruschweiler R, Griesinger C (2002) Model-free analysis of protein backbone motion from residual dipolar couplings. J Am Chem Soc 124:5822–5833
Prestegard JH, Al-Hashimi HM, Tolman JR (2000) NMR structures of biomolecules using field oriented media and residual dipolar couplings. Q Rev Biophys 33:371–424
Rexroth A, Schmidt P, Szalma S, Geppert T, Schwalbe H, Griesinger C (1995) New principle for the determination of coupling-constants that largely suppresses differential relaxation effects. J Am Chem Soc 117:10389–10390
Sass H-J, Musco G, Stahl SJ, Wingfield PT, Grzesiek S (2000) Solution NMR of proteins within polyacrylamide gels: diffusional properties and residual alignment by mechanical stress or embedding of oriented purple membranes. J Biomol NMR 18:303–309
Shaka AJ, Keler J, Freeman R (1983) Evaluation of a new broadband decoupling sequence: WALTZ-16. J Mag Res 53:313–340
Tian F, Bolon PJ, Prestegard JH (1999) Intensity-based measurement of homonuclear residual dipolar couplings from CT-COSY. J Am Chem Soc 121:7712–7713
Tian F, Fowler CA, Zartler ER, Jenney FA, Adams MW, Prestegard JH (2000) Direct measurement of 1H–1H dipolar couplings in proteins: a complement to traditional NOE measurements. J Biomol NMR 18:23–31
Tjandra N, Bax A (1997) Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 278:1111–1114
Tjandra N, Marquardt J, Clore GM (2000) Direct refinement against proton–proton dipolar couplings in NMR structure determination of macromolecules. J Magn Reson 142:393–396
Tolman JR (2002) A novel approach to the retrieval of structural and dynamic information from residual dipolar couplings using several oriented media in biomolecular NMR spectroscopy. J Am Chem Soc 124:12020–12030
Tolman JR, Ruan K (2006) NMR residual dipolar couplings as probes of biomolecular dynamics. Chem Rev 106:1720–1736
Tolman JR, Al-Hashimi HM, Kay LE, Prestegard JH (2001) Structural and dynamic analysis of residual dipolar coupling data for proteins. J Am Chem Soc 123:1416–1424
Tycko R, Blanco FJ, Ishii Y (2000) Alignment of biopolymers in strained gels: a new way to create detectable dipole–dipole couplings in high-resolution biomolecular NMR. J Am Chem Soc 122:9340–9341
Ulmer TS, Ramirez BE, Delaglio F, Bax A (2003) Evaluation of backbone proton positions and dynamics in a small protein by liquid crystal NMR spectroscopy. J Am Chem Soc 125:9179–9191
Vogeli B, Ying JF, Grishaev A, Bax A (2007) Limits on variations in protein backbone dynamics from precise measurements of scalar couplings. J Am Chem Soc 129:9377–9385
Vuister GW, Bax A (1994) Measurement of four-bond HN–Hα J-couplings in staphylococcal nuclease. J Biomol NMR 4:193–200
Wang AC, Bax A (1996) Determination of the backbone dihedral angles phi in human ubiquitin from reparametrized empirical Karplus equations. J Am Chem Soc 118:2483–2494
Wu Z, Delaglio F, Tjandra N, Zhurkin VB, Bax A (2003) Overall structure and sugar dynamics of a DNA dodecamer from homo- and heteronuclear dipolar couplings and 31P chemical shift anisotropy. J Biomol NMR 26:297–315
Yao L, Vogeli B, Torchia DA and Bax A (2008) Simultaneous NMR study of protein structure and dynamics using conservative mutagenesis. J Phys Chem A. doi:10.121/jp0772124
Zhang Q, Sun XY, Watt ED, Al-Hashimi HM (2006) Resolving the motional modes that code for RNA adaptation. Science 311:653–656
Zweckstetter M, Bax A (2002) Evaluation of uncertainty in alignment tensors obtained from dipolar couplings. J Biomol NMR 23:127–137
Acknowledgment
We thank Drs. Lisa Parsons for help in sample preparation, and Dennis A. Torchia and G. Marius Clore for helpful discussions. Financial support was obtained from the Swiss National Science Foundation (to B.V.). This work was supported by the Intramural Research Program of the NIDDK, NIH, and by the Intramural AIDS-Targeted Antiviral Program of the Office of the Director, NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Vögeli, B., Yao, L. & Bax, A. Protein backbone motions viewed by intraresidue and sequential HN–Hα residual dipolar couplings. J Biomol NMR 41, 17–28 (2008). https://doi.org/10.1007/s10858-008-9237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-008-9237-3