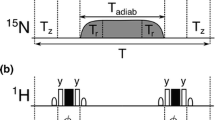
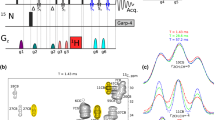
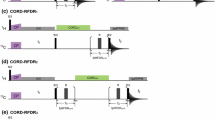
Abstract
High signal to noise is a necessity for the quantification of NMR spectral parameters to be translated into accurate and precise restraints on protein structure and dynamics. An important source of long-range structural information is obtained from 1H–1H residual dipolar couplings (RDCs) measured for weakly aligned molecules. For sensitivity reasons, such measurements are generally performed on highly deuterated protein samples. Here we show that high sensitivity is also obtained for protonated protein samples if the pulse schemes are optimized in terms of longitudinal relaxation efficiency and J-mismatch compensated coherence transfer. The new sensitivity-optimized quantitative J-correlation experiment yields important signal gains reaching factors of 1.5 to 8 for individual correlation peaks when compared to previously proposed pulse schemes.





Similar content being viewed by others
References
Andersson P, Weigelt J, Otting G (1998) Spin-state selection filters for the measurement of heteronuclear one-bond coupling constants. J Biomol NMR 12:435–441
Atreya HS, Szyperski T (2004) G-matrix Fourier transform NMR spectroscopy for complete protein resonance assignment. Proc Natl Acad Sci USA 101:9642–9647
Bax A, Vuister GW, Grzesiek S, Delaglio F, Wang AC, Tschudin R, Zhu G (1994) Measurement of homo- and heteronuclear J couplings from quantitative J correlation. Methods Enzymol 239, Pt C:79–105
Beraud S, Bersch B, Brutscher B, Gans P, Barras F, Blackledge M (2002) Direct structure determination using residual dipolar couplings: reaction-site conformation of methionine sulfoxide reductase in solution. J Am Chem Soc 124:13709–13715
Blackledge M (2005) Recent progress in the study of biomolecular structure and dynamics in solution from residual dipolar couplings. Prog Nucl Magn Reson 46:23–61
Boisbouvier J, Delaglio F, Bax A (2003) Direct observation of dipolar couplings between distant protons in weekly aligned nucleic acids. Proc Natl Acad Sci USA 100:11333–11338
Bouvignies G, Bernado P, Meier S, Cho K, Grzesiek S, Brüschweiler R, Blackledge M (2005) Identification of slow correlated motions in proteins using residual dipolar and hydrogen-bond scalar couplings. Proc Natl Acad Sci USA 102:13885–13890
Bouvignies G, Meier S, Grzesiek S, Blackledge M (2006) Ultrahigh-resolution backbone structure of perdeuterated protein GB1 using residual dipolar couplings from two alignment media. Angew Chem Int Ed Engl 45:8166–8169
Diercks T, Daniels M, Kaptein R (2005) Extended flip-back schemes for sensitivity enhancement in multidimensional HSQC-type out-and-back experiments. J Biomol NMR 33:243–259
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141
Hus JC, Marion D, Blackledge M (2001) Determination of protein backbone structure using only residual dipolar couplings. J Am Chem Soc 123:1541–1542
Kontaxis G, Delaglio F, Bax A (2005) Molecular fragment replacement approach to protein structure determination by chemical shift and dipolar homology database mining. Methods Enzymol 394:42–78
Kupce E, Boyd J, Campbell ID (1995) Short selective pulses for biochemical applications. J Magn Reson B 106:300–303
Lakomek NA, Fares C, Becker S, Carlomagno T, Meiler J, Griesinger C (2005) Side-chain orientation and hydrogen-bonding imprint supra-tau(c) motion on the protein backbone of ubiquitin. Angew Chem Int Ed Engl 44:7776–7778
Meier S, Haussinger D, Jensen P, Rogowski M, Grzesiek S (2003) High-accuracy residual 1HN–13C and 1HN–1HN dipolar couplings in perdeuterated proteins. J Am Chem Soc 125:44–45
Meiler J, Prompers JJ, Peti W, Griesinger C, Brüschweiler R (2001) Model-free approach to the dynamic interpretation of residual dipolar couplings in globular proteins. J Am Chem Soc 123:6098–6107
Nielsen NC, Bildsoe H, Jakobsen HJ, Sorensen OW (1989) Composite refocusing sequences and their application for sensitivity enhancement and multiplicity filtration in INEPT and 2D correlation spectroscopy. J Magn Reson 85:359–380
Ottiger M, Delaglio F, Bax A (1998) Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson 131:373–378
Pervushin K, Vogeli B, Eletsky A (2002) Longitudinal (1)H relaxation optimization in TROSY NMR spectroscopy. J Am Chem Soc 124:12898–12902
Piotto M, Saudek V, Sklenar V (1992) Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR 2:661–665
Prestegard JH, Bougault CM, Kishore AI (2004) Residual dipolar couplings in structure determination of biomolecules. Chem Rev 104:3519–3540
Schanda P, Brutscher B (2005) Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J Am Chem Soc 127:8014–8015
Schanda P, Brutscher B (2006) Hadamard frequency-encoded SOFAST-HMQC for ultrafast two-dimensional protein NMR. J Magn Reson 178:334–339
Schanda P, Kupce E, Brutscher B (2005) SOFAST-HMQC experiments for recording two-dimensional heteronculear correlation spectra of proteins within a few seconds. J Biomol NMR 33:199–211
Schanda P, Forge V, Brutscher B (2006a) HET-SOFAST NMR for fast detection of structural compactness and heterogeneity along polypeptide chains. Magn Reson Chem 44:S177–S184
Schanda P, van Melckebeke H, Brutscher B (2006b) Speeding up three-dimensional protein NMR experiments to a few minutes. J Am Chem Soc 128:9042–9043
Sibille N, Blackledge M, Brutscher B, Coves J, Bersch B (2005) Solution structure of the sulfite reductase flavodoxin-like domain from Escherichia coli. Biochemistry 44:9086–9095
Smith MA, Hu H, Shaka AJ (2001) Improved broadband inversion performance for NMR in liquids. J Magn Reson 151:269–283
Tjandra N, Bax A (1997) Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 278:1111–1114
Wimperis S, Bodenhausen G (1986) Heteronuclear coherence transfer over a range of coupling constants. A broadband-INEPT experiment. J Magn Reson 69:264–282
Wu ZR, Bax A (2002) Measurement of long-range 1H–1H dipolar couplings in weakly aligned proteins. J Am Chem Soc 124:9672–9673
Acknowledgments
This work was supported by the Commissariat à l’Energie Atomique, the Centre National de la Recherche Scientifique, the French Research Agency (ANR), Human Frontier Science Program Organization and the European Commission (EU-NMR). P.S. and R.S. acknowledge support from the French ministry of education, research, and technology. We thank Beate Bersch, Isabel Ayala, and Jacques Covès (IBS Grenoble) for the preparation of the isotope-labeled protein samples.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Paul Schanda and Ewen Lescop contributed equally to this work.
Rights and permissions
About this article
Cite this article
Schanda, P., Lescop, E., Falge, M. et al. Sensitivity-optimized experiment for the measurement of residual dipolar couplings between amide protons. J Biomol NMR 38, 47–55 (2007). https://doi.org/10.1007/s10858-006-9138-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-006-9138-2