Abstract
Based on the concept of tissue engineering (Cells—Scaffold—Bioactive molecules), regenerative endodontics appeared as a new notion for dental endodontic treatment. Its approaches aim to preserve dental pulp vitality (pulp capping) or to regenerate a vascularized pulp-like tissue inside necrotic root canals by cell homing. To improve the methods of tissue engineering for pulp regeneration, numerous studies using in vitro, ex vivo, and in vivo models have been performed. This review explores the evolution of laboratory models used in such studies and classifies them according to different criteria. It starts from the initial two–dimensional in vitro models that allowed characterization of stem cell behavior, through 3D culture matrices combined with dental tissue and finally arrives at the more challenging ex vivo and in vivo models. The travel which follows the elaboration of such models reveals the difficulty in establishing reproducible laboratory models for dental pulp regeneration. The development of well-established protocols and new laboratory ex vivo and in vivo models in the field of pulp regeneration would lead to consistent results, reduction of animal experimentation, and facilitation of the translation to clinical practice.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Following the Quality Clinical Guidelines for regenerative endodontic therapy published by the European Society of Endodontology (ESE) and by the American Association of Endodontists (AAE) (Table 1) [1] dental pulp regeneration approaches comprise two types of clinical procedures - Vital Pulp Therapies (VPT) that address vital teeth or Regenerative Endodontic Procedures (REP) of necrotic teeth. Pulp capping is a VPT applied when the healthy dental pulp has been minimally exposed. In this case, the direct application of a bioactive material would induce tissue response and regeneration preserving tooth vitality. The second regenerative procedure also known as “revascularization” is applied to necrotic teeth. It is a biological approach based on blood colonization of disinfected empty root canals by inducing periapical bleeding. The formed blood clot inside the canal acts as a scaffold containing growth factors from blood and dentin walls and undifferentiated cells from the periapical region which have migrated by “cell homing”.
The beginning of pulp regeneration research was marked by the introduction of stem cells of dental origin [2]. The tool to describe these cells was classical 2D in vitro cell culture. However, these models did not simulate cell interaction with an extracellular matrix (ECM), so 3D in vitro models consisting of cell culture on a 3D matrix were introduced to evaluate cell-scaffold interactions [3, 4]. These models have been applied to test the effects of different scaffolds or bioactive molecules [5, 6]. Nevertheless, such in vitro models are far from reality and they do not guarantee adequate translation in clinical practice. The necessity of studies closer to real conditions led to the development of ingenious ex vivo models such as dentin-pulp slices [7], the entire tooth or crown culture [8, 9], and the mandible slice culture [10] to study pulp capping procedures, pulpitis [11, 12] or scaffolds for pulp regeneration [10].
The need to establish representative in vitro and ex vivo models for dental pulp regeneration resulted in the development of several in vivo experiments, making them the most widely spread models. Two categories of animal models were described: ectopic and orthotopic models. Ectopic models are farther from reality since they use Teflon tubes, dentin slices, or roots, containing a testing sample to be placed subcutaneously in the rodent’s back [13]. Orthotopic models are closer to clinical reality, the scaffolds and cells are placed in the pulp chambers or root canals of dogs [14]; porcine [15]; ferrets [16], or sheep [17].
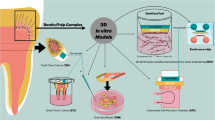
Hence a vast number of models for regenerative endodontics has been published and choosing one could be quite challenging when designing a study. Therefore with this narrative bibliographic review aimed to present and analyze in vitro, ex vivo, and in vivo models for dental pulp regeneration to help with the choice of a model (Fig. 1).
2 Materials and methods
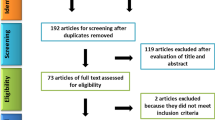
Analyzed articles were selected from Pubmed/Medline database following Problem, Intervention, Comparison, Outcome, Study (PICOS) criteria [18], where: P- regenerative endodontics, I- vital pulp capping for vital teeth, or regenerative endodontic procedure for necrotic teeth, C- comparison between study design using different cell lines, growth factors or scaffolds, O- dental origin cell behavior, pulp-like tissue formation, and S- in vitro, ex vivo, in vivo studies.
Exclusion criteria: randomized clinical trials, non-randomized clinical trials, prospective cohort studies, clinical cases, and articles whose methodology was inadequately explained.
3 In vitro models for pulp regeneration
In vitro models used cells or biological molecules isolated from their natural environment to carry out experimentation outside a living organism in controlled laboratory conditions. They offer more detailed information about biological phenomena than studies in whole organisms. Besides, they are fast, cheap, and allow multiple experiments with a large number of samples thus reducing the number of in vivo studies/subjects needed [19]. However, extrapolation to humans is difficult and they lack data about biokinetics [20, 21].
Cell culturing in in vitro models can be performed in two-dimensional (2D) or tri-dimensional (3D) conditions. 2D models are used to describe cell behavior such as adhesion, proliferation, and differentiation and also to study the cytotoxicity of bioactive molecules. However, they are not adequate for studying tissue regeneration because they lack the interaction of tested components with the ECM.
To get over these disadvantages, 3D models have been developed; such as cellular spheroids, cell-laden hydrogels, mini-organs, and microfluidic organs-on-a-chip [22]. These 3D models better represent cell environments, allowing evaluation of the efficacy and safety of biochemical agents and modeling of biological processes [23]. A disadvantage of these models is the impossibility to mimic the interaction between ECM and different cell types [24]. In vitro models are summarized in Table 2.
3.1 In vitro models for cell behavior evaluation
Characterization of stem cells from dental origin has been achieved through 2D models. They allow different sources of cells involved in pulp regeneration processes to be described as cells from the dental pulp tissue (DPSC) [2, 25]; stem cells from the Apical Papilla (SCAP) of immature teeth, or from the Periodontal Ligament (PDLSC) [26, 27]. Mesenchymal Stem Cells (MSC) have been detected in the periapical zone so it is pertinent to consider them in pulp regeneration studies [28, 29].
All these cells have the potential to differentiate into odontoblasts, adipocytes, or neuron-like cells by culturing them in different media [30,31,32]. For odonto-osteogenic differentiation, basal media can be enriched with BMP-4, L-ascorbate-2-phosphate, dexamethasone, and phosphate [25]. Adipocyte-inducing medium is obtained by mixing insulin, isobutylmethylxanthine, and dexamethasone [33]. For neural differentiation, the neurobasal medium is supplemented with B27, epidermal growth factor, or fibroblast growth factor (FGF) [25, 34].
Later, 2D models conceived on dentin slices have been used to evaluate cell behavior on dentin surfaces. Dentin slices of human teeth were sterilized, placed on culture plates, and seeded with dental stem cells to evaluate adhesion, proliferation, and differentiation [35]. The advantage of this model is the use of dentin which represents a natural tooth tissue substrate and resembles clinical situations more than plastic plates. Moreover, it allows the evaluation of the effect of growth factors naturally trapped in the dentin collagen network. However, sterilization leads to the degradation of the bioactive factors. Other chemical treatments such as sodium hypochlorite or EDTA 17%, should be prioritized since they are considered to be less aggressive against bioactive molecules. Nevertheless, cell deposition in a liquid instead of in a 3D matrix on a dentin surface moves this model away from natural tooth conditions.
3.2 In vitro models for scaffold evaluation
Accurate laboratory models are necessary to evaluate dental stem cell interactions in 3D matrices used as scaffolds for pulp regeneration since characteristics of cell environment such as surface chemistry, topography, and elastic modulus of the matrix influence local signals critical for cell behavior as survival, self-renewal, mobilization, proliferation and differentiation [36, 37].
3.2.1 3D Matrices as scaffolds
3D matrices show beneficial effects on dental stem cell metabolism and differentiation because they provide a conducive environment in which dental repair can occur [38,39,40]. Hydrogels are the most recently adopted biomaterials as scaffolds in pulp regeneration since they are easy to apply, cheap and they have good mechanical and chemical properties [41]. The most widespread materials with beneficial properties for cell survival and proliferation are fibrin or collagen-based materials (Matrigel, Puramatrix) among others [38, 42,43,44,45,46]. These matrices are obtained by the polymerization of a solution containing cells or biomolecules that is injected inside the root canal where it jellifies. However, the polymerization process could be hazardous and entails dimensional changes.
To overcome these disadvantages innovative methods for hydrogel preparation as 3D bioprinting were applied. The technique relies on laser and cell-laden materials as ink to print fibers that could be placed inside the root canal to support the formation of pericyte microvascular networks [47]. Nonetheless, adaptation to root canal walls may not be good enough.
Out of the need to overcome this problem, a novel approach to bioprinting arose: The Light (Lithography) and Digital Light Processing (DLP) of a photo-crosslinked hydrogel that supports cell survival. The material is to be injected into the empty root canal and polymerized with a dental curing light [48]. It does not have the biochemical and biomechanical characteristics of the human dental pulp to induce cell metabolism and new tissue formation of a pulp-like tissue. That is why other types of materials have been proposed, such as the dental pulp decellularized ECM, alone or combined with biomaterials such as alginate [39, 49,50,51]. This scaffold supports the odontogenic differentiation of different cell types (BMSC, DPSC, and PDLSC) with no need of adding inductive biomolecules [52]. Results reflect the advantage of pulp ECM due to the presence of biochemical and biomechanical cues that render it a more physiologic environment for cells. However, the difficulty of this technique resides in the extirpation of a human dental pulp and shape adaptation to root canal walls.
3.2.2 3D matrices on dental tissue substrate as a scaffold
To better mimic the dental pulp environment and assess the interaction between cells-scaffold and dental tissue, the complexity of the models increases. For instance, the use of dentin slices with a hydrogel containing cells was proposed to prove cell adhesion, survival, and proliferation that happen in contact with dentin [38, 44, 53].
An interesting approach is the full-length root canal model. It consists of constructed pre-vascularized root canals using a cell-laden hydrogel (gelatin methacryloyl) with encapsulated odontoblasts to obtain blood capillaries inside full-length root canals [54]. Human root fragments 9 mm long and with an apical diameter of 1.5 mm are sterilized, endodontically treated, rinsed with EDTA17%, and sectioned longitudinally in two parts to be re-attached. A prefabricated fiber is longitudinally positioned inside the canal and the hydrogel with cells is loaded into it and photopolymerized. Later, the central fiber is taken out, leaving a microchannel inside the hydrogel. Finally, endothelial cells are injected inside the channel resulting in a pre-vascularized full-length dental pulp-like tissue construct.
3.3 In vitro models for bioactive molecules evaluation
Bioactive molecules are extensively applied in laboratory experiments due to their capacity to guide the healing process in pulp capping procedures and to induce cell homing in REP of necrotic teeth. They can be natural molecules as growth factors (GF), or chemical products present in biomaterials. To facilitate the description of in vitro models for pulp regeneration we classified biomolecules based on their application in two groups (Table 2).
-
Evaluation of the chemotactic effect of natural biomolecules
-
Evaluation of cytotoxicity and biocompatibility of biomolecules
3.4 Chemotactic effect of natural biomolecules
Chemotactic effect of natural biomolecules as GF is necessary for cell-homing approaches, based on periapical cell migration inside the root canal space of a necrotic tooth. 2D in vitro cell cultures in plastic dishes have been used to analyze the influence of several GF (VEGF, BMP-2, FGF, TGF, CGF) in dental stem cell differentiation [55,56,57]. These studies allow the description of cell behaviors under the effect of biomolecules. However, they do not represent an environment close to reality since cells are cultured on a plastic substrate. The same procedures have been carried out in dentin slices or powder to prove the potential of dentin conditioning agents used in clinical procedures such as 17% EDTA, 10% citric acid, 1% phytic acid, or 37% phosphoric acid to release dentin GF and their beneficial effects regarding cell survival and proliferation [58,59,60].
Another 2D model used to assess the chemotactic effect of dentin GF is the Boyden chamber. It is a suspended hollow plastic chamber over a larger well. Both compartments are separated by a porous membrane. Cells are placed inside the upper chamber, and they could migrate through the membrane if the bioactive factors placed in the lower chamber have a cell mobilizing potential. One study used this chamber to assess liposomal delivery of encapsulated Demineralized Dentin Matrix, VEGF, and TGF-β1 proving that GFs from demineralized dentin matrix can recruit and promote odonto-differentiation of DPSC [61]. Further, the Boyden chamber was adapted to prove the release by EDTA of dentin chemotactic GF [62]. With this aim, EDTA pre-treated dentin discs were placed in the lower chamber and the migration of cells to non-treated and treated dentin was compared. Results proved that dentin released GFs trapped on the dentin matrix, enhancing cell migration.
Another study described an in vitro model consisting of root fragments treated by the conditioning agents mentioned above, to describe cell adhesion and morphology of adipose-derived MSCs attached to dentin [63]. The disadvantage of this model is the lack of a 3D matrix containing cells. A classical in vitro model with a culture plate with DPSC in a 3D matrix (Matrigel) has been performed to prove that VEGF and CGF enhanced cell proliferation, migration, and differentiation [49, 64]. Another study developed a more complex model based on the injection of a scaffold charged with cells inside root canal fragments pre-treated with different conditioning agents [65]. In this study, SCAPs were mixed with platelet rich-plasma and cultured into the root for 21 days. Results proved that irrigants such as EDTA 17% enhanced SCAP proliferation which could be beneficial in regenerative procedures. This model could be considered appropriate for studying the chemotactic effect of natural biomolecules since it represents a 3D matrix containing cells inside a natural root canal and it respects conditions such as hypoxia.
3.5 Cytotoxicity and biocompatibility of biomolecules
To test cytotoxicity and biocompatibility of biomolecules and biomaterials the classical ISO cell test is applied [66]. It consists of the incubation of cells with biomolecules or biomaterials (cytotoxicity) or with a culture medium conditioned by the evaluated biomaterial (biocompatibility). This model is practical, cheap, and easy to develop giving clear results concerning cell biocompatibility.
More complex bi-dimensional devices have been developed, such as the in vitro pulp chamber model. This approach is useful to test the cytotoxicity of biomaterials based on the perfusion of molecules into dentinal tubes. This device is made of two chambers separated by a dentin slice. The upper chamber contains the tested biomaterial or bioactive molecules and the lower chamber contains dental cells on a coverslip [67, 68]. This 2D model was adapted to be used in 3D conditions. It consists of the classical in vitro pulp chamber but with cells cultured in a 3D matrix made of polyamide meshes [69].
The most recent innovation in the field of 3D in vitro models to evaluate bioactive molecules is the tooth-on-a-chip device. It is a microphysiologic platform that mimics conditions of the pulp–dentin interface with biomaterials and enables live-cell imaging to study dental pulp-cell response to biomaterials [70]. This microdevice is made by two accessible chambers. One represents the pulp side and the other the cavity with the tested material. The interface reproduces the interface of pulp with dentin-material. The advantage of this miniaturized organ system is that it replicates levels of tissue functionality difficult to achieve with conventional 2D or 3D cell culture models and it also avoids multifactorial challenges that cannot be controlled in vivo [71, 72].
4 Ex vivo models for pulp regeneration
By definition, ex vivo models represent a recently extracted entire tissue or organ with minimal alteration from its natural state cultured to preserve vitality under laboratory conditions. These models aim to describe cellular and physiological processes in an environment similar to conditions in a real organ or tissue. Since cytoarchitecture and intercellular connections with ECM are maintained, metabolic processes are closer to the in vivo state than an in vitro model [73].
In the regenerative endodontic field, this kind of laboratory models has been applied to test pulp-capping biomaterials [74, 75]. However, even if these models better mimic clinical situations, they are unsuitable to study regenerative endodontic procedures of necrotic teeth by cell homing through the evaluation of a scaffold inside the root canal. The reason is that to consider a model as ex vivo it should include the whole alive tissue or organ with all the cell types with no or minimal modification. If we translate this to dental pulp regeneration, it means that we should keep intact pulp inside the root. Since dental pulp should be removed to place the scaffold inside the empty canal space, we should not consider root canals with matrices as real ex vivo models. Nevertheless, one study overcame this barrier by using a mandible slice organ culture model to implant a Multidomain Peptide Hydrogel scaffold in the core of intact dental pulp [10]. Ex vivo models for pulp regeneration are presented in Table 3.
4.1 Evaluation of cell behavior in ex vivo models
4.1.1 Cell behavior in normal conditions
As mentioned before, studies that use endodontically treated root canal slices could not be considered ex vivo models because pulp tissue and cells are not conserved. Controversially, some studies employ the organ culture system to study the ultrastructure of the odontoblast [76, 77]. With this aim, incisors were extracted and cut longitudinally, and dental pulp was carefully extracted without damaging the odontoblast layer attached to the dentin. Teeth were conserved in culture media and cells were proven to remain alive. This study could be considered as a borderline between in vitro and ex vivo since it did not conserve pulp integrity but a layer of cells corresponding to it.
Pure ex vivo studies have been carried out to find adequate culture conditions to preserve the viability and function of the pulp tissue. This culture method is useful to study the physiological function of odontoblasts and describe dental pulp homeostasis and cellular behavior. With this aim, Hasegawa developed a pulp-dentin slice culture system in rats. He proved that a rocking culture with higher Oxygen levels (95% O2) was more favorable than a hyperbaric stationary culture to maintain cell viability [78].
Later, another study described an ex vivo model based also on the culture of dentin-pulp slices of rat incisors but embedded in an agar-based medium and cultured on floating Millipore filters in Trowel-type cultures. Dental pulp showed no inflammation and preserved vitality for up to 2 weeks [79].
4.1.2 Cell behavior after pulp injury-inflammatory conditions
Magliore et al. evaluated pulp tissue response after dentin drilling exposure [80]. They used thick slices of human teeth drilled immediately after extraction and cultured from 3 days to 1 month. They proved that the exposed pulp showed healing aspects such as cell proliferation, neovascularization, and the presence of functional cuboidal cells close to the injured area. Murray et al. presented a model to measure and compare the responses of pulp tissue to cavity preparation and restoration. They examined variables such as the preparation method, remaining dentin thickness, drill speed, conditioning with EDTA, and different filling materials [81]. However, these models do not respect the natural hypoxia present in human dental pulp where oxygen only enters through the apical foramen—a condition that could induce a change in cell metabolism.
Later, Tecles et al. developed an entire tooth culture system to describe the inflammatory reaction and stem cell migration of dental pulp after simulating a clinical exposure [82, 83]. They used entire immature third molars recently extracted to make cavities with or without pulp exposure. Subsequently, the tooth crowns were fixed to the covers of the culture plates, leaving the apical parts floating inside the culture media for up to 4 weeks. This model respects the hypoxia and natural environment of the dental pulp, making it the most advantageous model to evaluate dental pulp response to injury. However, its application has not been translated to pulp revascularization procedures.
Besides, none of these models consider usual conditions in real life as the presence of affected tissue and bacteria that avoid favorable cell response and pulp regeneration. This was proved by an animal model in dogs [84].
4.2 Evaluation of scaffolds for regenerative endodontics
An ex vivo model of mandible slice organ culture was performed to inject in the core of an intact pulp a Multidomain Peptide Hydrogel (MDP) scaffold. Mandibles were dissected, and soft tissues were removed and cut into slices of 2 mm. MDP scaffolds were injected in columns through the entire length of the incisor dental pulp core. Slices were cultured for up to 10 days and histologically evaluated [10]. Results confirmed the biocompatibility of the scaffold and preservation of the surrounding tissue architecture. This model fits into the definition of ex vivo since it keeps all intraoral tissues (bone, periodontal, teeth, and dental pulp). Despite the lack of hypoxia, since the culture is performed in slices of 2 mm instead of along the entire tooth, we consider it the most pertinent model so far for the study of scaffolds for pulp regeneration.
4.3 Evaluation of bioactive molecules and biomaterials for pulp capping
Two types of ex vivo systems related to pulp capping procedures exist the tooth slices system [74], and the entire tooth culture system [8].
Dentin-pulp slices model has been employed to evaluate the responses of human pulp to direct capping with resin adhesive systems, calcium hydroxide, composite resins, or bioceramic types of cement [7, 85, 86]. It has also been used to evaluate pulp reaction to molecules such as Iloprost or VEGF and prove their angiogenic potential by adding them to culture media [7, 87]. Nevertheless, it does not reflect the hypoxia conditions of the human dental pulp.
Facing this disadvantage, the entire tooth culture system has been proposed to evaluate dental pulp response after the application of bioceramic cements in pulp capping procedures [11, 74]. This method also proved pulp healing when collagen or MTA charged with nano plexes of polyethyleneimine and plasmid DNA encoding for FGF-2 and BMP-2 were used for 14 days [88]. This model preserves the natural environment of the dental pulp, making it the closest to reality to evaluating dental pulp response during pulp capping procedures. Ex vivo studies that evaluate the migration potential of bioactive molecules used in cell homing have not been found in the literature.
5 In vivo models for pulp regeneration
Animal experiments provide important information about the mechanical behavior of used biomaterials and about their efficacy and biocompatibility [89]. They can be classified into two categories: orthotopic and ectopic models.
5.1 Ectopic models
In ectopic models, the implantation occurs in an abnormal position. These models have been widely used for pulp regeneration due to their low cost and because they are easy to handle (Table 4). Animals that best fit this model are small species such as rodents [90]. Following the objective, we divided these models into two groups. The first group includes models where the scaffolds are implanted subcutaneously in a dorsal zone to evaluate the behavior of dental stem cells and scaffolds. Scaffolds such as poly(lactic-co-glycolic) in rabbits [91] or collagen in mice have been tested [92]. Results demonstrated the organization of newly derived pulp-like tissue.
The second group involves the implantation of more complex systems made by the scaffold placed in a carrier (dentin or root slices) to evaluate the complete biological response. First, studies that implanted tooth slices [93,94,95,96,97,98,99]. Results demonstrated osteoblastic, odontoblastic, cementoblastic, and fibroblastic differentiation.
More complex models consist of implanting a Teflon tube or dental root with the tested biomaterial in the canal subcutaneously [13, 100,101,102,103]. One end of this graft could be sealed with a bioactive cement, as MTA or Biodentine, or not whereas the other remains open to allow cells and blood to enter. This model has been performed to test cell-free approaches [104] as well as a cell-based therapy for REP proving endodontic space revitalization and pulp-like tissue formation. It was also used to compare the regenerative properties of human stem cells of the apical papilla (SCAPs) seeded in platelet-rich plasma (PRP) as a scaffold [105].
Kodonas et al. performed an ectopic mini-pig model, by implanting inside a post-extraction socket of the jawbone a root fraction with cells and scaffold inside the canal, they prove cell organization and new matrix formation [106].
5.2 Orthotopic models
Orthotopic transplantation is based on the implantation of a graft in its natural location. These models are hazardous due to the necessity to use big animals, rendering them expensive and difficult to manage. However, they have been widely used in the pulp regeneration field because they objectively represent a clinical situation in functional teeth. The method consists in performing a regenerative endodontic treatment in the chosen animal while testing a new technique or material. The protocol for tooth preparation should follow clinical guidelines already established for these procedures. These models are based on the introduction of external cells (cell-based therapy) or cell-free REP (cell homing) (Table 5).
Many animal species have been standardized to carry out these models. Rodents are not commonly used because they are too small to perform an endodontic treatment on them. However, by using endodontic microscopes, a study evaluated tissue formation after classical REP under photobiomodulation therapy in rats assessing pulp-like tissue formation [90] or in chimeric mice [107].
Dogs are widely used for orthotopic transplantation since their teeth show similar growth patterns and pathophysiology to humans [108]. However, they cannot be considered an ideal model due to differences in the apical region [109]. Nonetheless, several studies testing cell-based therapy that obtained regeneration of pulp-like tissue were performed [14, 96, 110, 111]. For cell homing approach dog models recreating apical periodontitis have been used with blood clots alone or in combination with scaffolds as chitosan hydrogels [111] or collagen [112]. Negative influence in pulp regeneration of important factors were considered by some authors, as advanced age [14] or inflammation due to accidental over instrumentation [84].
In the study mentioned above, non-inflamed samples showed signs of repair, contrary to inflamed canals, proving the importance of controlling periapical inflammation to achieve dental pulp regeneration. This could be explained by the high level of pro-inflammatory macrophages that avoid favorable cell response and pulp regeneration. Although, these cells could be shifted to anti-inflammatory, by reducing bacteria concentration, and enhancing the tissue remodeling process [113].
Therefore, significant attention has been devoted to porcine species due to their similarities with humans. Disadvantages are the high growth rate and excessive weight being difficult to handle.
Cell homing studies obtained and pulp-like tissue formation in mini-pigs [114]. Other studies carried out in a miniature swine model obtained vascularized pulp-like tissue with a layer of dentin-like tissue along the canal walls [15, 115]. However, the disadvantages of using pigs mentioned before remained in this model. Ferrets have also been used to test cell-based pulp regeneration approaches as REP since their teeth anatomy, physiology, histology, and pathology are similar to humans, obtaining pulp-like tissue formation [16, 116].
Sheep have been used for REP by cell homing due to similarities with human dentition, besides being widely available, easy to handle, and cheap compared to other big species [117]. One study examined the response of immature sheep teeth, whose pulp was exposed and infected, and treated within 4 weeks following the classical revitalization protocol with or without collagen as a scaffold. Results assessed root maturation and dentin wall thickening [17].
6 Conclusion
6.1 In vitro, ex vivo, and in vivo studies allow a better understanding of regenerative endodontics procedures
Thanks to their development, new cell lines, molecules and matrices are proposed resulting in new approaches to obtain a real pulp regeneration. At in vitro and in ex vivo conditions this regeneration seems to be successful. But in in vivo models the results are closer to clinical conditions and some factors related to the living organism must be taken into consideration.
Pulp capping procedures are in an advanced stage since complex in vitro and ex vivo models close to reality exist allowing representative results that could be easily translated to in vivo evaluation and consequently to clinical practice.
On the other hand, REP of necrotic teeth is insufficiently developed due to the wide utilization of in vivo models that lack reproducibility and predictability because of variability between animals and systemic factors. Future directions include the development of controlled protocols for laboratory models that better simulate REP of necrotic teeth respecting clinical conditions, for example, the presence of inflammatory tissue. Special attention should be paid to the evolution of reproducible ex vivo models that allow a realistic representation of the clinical situation. This would allow us to accurately study different factors such as scaffolds, cells, bioactive molecules, and variations in the clinical situation and protocols to achieve consistent results. Then, animal experimentation could be reduced and translation into clinical practice would be faster and safer.
References
Duncan HF, Galler KM, Tomson PL, Simon S, El‐Karim I, Kundzina R, et al. European Society of Endodontology position statement: Revitalization procedures. Int Endod J. 2016;49:717–23. https://doi.org/10.1111/iej.12629
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000;97:13625–30. https://doi.org/10.1073/pnas.240309797
Eramo S, Natali A, Pinna R, Milia E. Dental pulp regeneration via cell homing. Int Endod J. 2018;51:405–19. https://doi.org/10.1111/iej.12868
Hadjichristou C, About I, Koidis P, Bakopoulou A. Advanced in vitro experimental models for tissue engineering-based reconstruction of a 3D dentin/pulp complex: a literature review. Stem Cell Rev Rep. 2021;17:785–802. https://doi.org/10.1007/s12015-020-10069-8
Orti V, Collart-Dutilleul PY, Piglionico S, Pall O, Cuisinier F, Panayotov I. Pulp regeneration concepts for nonvital teeth: from tissue engineering to clinical approaches. Tissue Eng Part B: Rev 2018;24:419–42. https://doi.org/10.1089/ten.teb.2018.0073
Gong T, Heng BC, Lo ECM, Zhang C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016;2016:1–13. https://doi.org/10.1155/2016/9204574
Goncalves S, Dong Z, Bramante C, Holland G, Smith A, Nor J. Tooth slice–based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811–4. https://doi.org/10.1016/j.joen.2007.03.012
Téclès O, Laurent P, Aubut V, About I. Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J Biomed Mater Res 2008;85B:180–7. https://doi.org/10.1002/jbm.b.30933
Botelho J, Cavacas MA, Borrecho G, Polido M, Oliveira P, Martins Dos Santos J. Human ex vivo dentin-pulp complex preservation in a full crown model. J Oral Biol Craniofac Res. 2017;7:19–22. https://doi.org/10.1016/j.jobcr.2016.12.002
Moore AN, Perez SC, Hartgerink JD, D’Souza RN, Colombo JS. Ex vivo modeling of multidomain peptide hydrogels with intact dental pulp. J Dent Res. 2015;94:1773–81. https://doi.org/10.1177/0022034515600380
Laurent P, Camps J, About I. BiodentineTM induces TGF-β1 release from human pulp cells and early dental pulp mineralization: Biodentine induces mineralization and TGF-β1 release. Int Endod J. 2012;45:439–48. https://doi.org/10.1111/j.1365-2591.2011.01995.x
Richert R, Ducret M, Alliot‐ Licht B, Bekhouche M, Gobert S, Farges J. A critical analysis of research methods and experimental models to study pulpitis. Int Endodontic J. Published online February 2, 2022:iej.13683. https://doi.org/10.1111/iej.13683
Rosa V, Zhang Z, Grande RHM, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013;92:970–5. https://doi.org/10.1177/0022034513505772
Iohara K, Murakami M, Nakata K, Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol. 2014;52:39–45. https://doi.org/10.1016/j.exger.2014.01.020
Zhu X, Liu J, Yu Z, et al. A miniature swine model for stem cell-based de novo regeneration of dental pulp and dentin-like tissue. Tissue Eng Part C: Methods. 2018;24:108–20. https://doi.org/10.1089/ten.tec.2017.0342
Verma P, Nosrat A, Kim JR, Price JB, Wang P, Bair E, et al. Effect of residual bacteria on the outcome of pulp regeneration in vivo. J Dent Res. 2017;96:100–6. https://doi.org/10.1177/0022034516671499
Altaii M, Cathro P, Broberg M, Richards L. Endodontic regeneration and tooth revitalization in immature infected sheep teeth. Int Endod J. 2017;50:480–91. https://doi.org/10.1111/iej.12645
Russell R, Chung M, Balk EM. Issues and Challenges in Conducting Systematic Reviews to Support Development of Nutrient Reference Values: Workshop Summary: Nutrition Research Series, Vol. 2. Rockville (MD): Agency for Healthcare Research and Quality (US); 2009. P. https://www.ncbi.nlm.nih.gov/books/NBK44088/ (TechnicalReviews, No. 17.2.) 2, Systematic Review Methods
Aslantürk ÖS. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In: Larramendy ML, Soloneski S, eds. Genotoxicity - A Predictable Risk to Our Actual World. InTech; 2018. https://doi.org/10.5772/intechopen.71923
Saeidnia S, Manayi A, Abdollahi M. From in vitro experiments to in vivo and clinical studies; pros and cons. CDDT. 2016;12:218–24. 10.2174/1570163813666160114093140
Winn LM. In vitro models in developmental toxicology. In: Hansen JM, Winn LM, (eds.) Developmental Toxicology. Vol 1965. Methods in Molecular Biology. New York: Springer; 2019. https://doi.org/10.1007/978-1-4939-9182-2_1 1–6.
Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. ASSAY Drug Dev Technol. 2014;12:207–18. https://doi.org/10.1089/adt.2014.573
Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater. 2018;3:21–37. https://doi.org/10.1038/s41578-018-0006-y
Pati F, Gantelius J, Svahn HA. 3D bioprinting of tissue/organ models. Angew Chem Int Ed. 2016;55:4650–65. https://doi.org/10.1002/anie.201505062
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12. https://doi.org/10.1073/pnas.0937635100
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. https://doi.org/10.1016/j.joen.2007.11.021
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364:149–55. https://doi.org/10.1016/S0140-6736(04)16627-0
Chrepa V, Henry MA, Daniel BJ, Diogenes A. Delivery of apical mesenchymal stem cells into root canals of mature teeth. J Dent Res. 2015;94:1653–9. https://doi.org/10.1177/0022034515596527
Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–8. https://doi.org/10.1016/j.joen.2010.10.009
Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. https://doi.org/10.1177/0022034509340867
Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13:152–9. https://doi.org/10.1007/s11684-018-0628-x
Aydin S, Şahin F. Stem Cells Derived from Dental Tissues. In: Turksen K, ed. Cell Biology and Translational Medicine, Volume 5. Vol 1144. Advances in Experimental Medicine and Biology. Springer International Publishing; 2019:123-32. https://doi.org/10.1007/5584_2018_333
Lai N, Sims JK, Jeon NL, Lee K. Adipocyte induction of preadipocyte differentiation in a gradient chamber. Tissue Eng Part C Methods. 2012;18:958–67. https://doi.org/10.1089/ten.TEC.2012.0168
Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, et al. Dental pulp stem cells: their potential in reinnervation and angiogenesis by using scaffolds. J Endod. 2017;43:S12–6. https://doi.org/10.1016/j.joen.2017.06.001
Messer RL, Davis CM, LewisJB, Adams Y, Wataha JC. Attachment of human epithelial cells andperiodontal ligament fibroblasts to tooth dentin. J Biomed Mater ResA. 2006;79:16–22. https://doi.org/10.1002/jbm.a.30703.
Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. https://doi.org/10.1038/nmat3339
Celiz AD, Smith JG, Langer R, Anderson DG, Winkler DA, Barrett DA, et al. Materials for stem cell factories of the future. Nat Mater. 2014;13:570–9. https://doi.org/10.1038/nmat3972
Cavalcanti BN, Zeitlin BD, Nör JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater. 2013;29:97–102. https://doi.org/10.1016/j.dental.2012.08.002
Smith JG, Smith AJ, Shelton RM, Cooper PR. Dental pulp cell behavior in biomimetic environments. J Dent Res. 2015;94:1552–9. https://doi.org/10.1177/0022034515599767
Jazayeri HE, Lee SM, Kuhn L, Fahimipour F, Tahriri M, Tayebi L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent Mater. 2020;36:e47–58. https://doi.org/10.1016/j.dental.2019.11.005
Abbass MMS, El-Rashidy AA, Sadek KM, Moshy SE, Radwan IA, Rady D, et al. Hydrogels and dentin–pulp complex regeneration: from the benchtop to clinical translation. Polymers. 2020;12:2935. https://doi.org/10.3390/polym12122935
Ducret M, Montembault A, Josse J, Pasdeloup M, Celle A, Benchrih R, et al. Design and characterization of a chitosan enriched fibrin hydrogel for human dental pulp regeneration. Dent Mater. 2019;35:523–33. https://doi.org/10.1016/j.dental.2019.01.018
Kwon YS, Lee SH, Hwang YC, Rosa V, Lee KW, Min KS. Behavior of human dental pulp cells cultured in a collagen hydrogel scaffold cross-linked with cinnamaldehyde. Int Endod J. 2017;50:58–66. https://doi.org/10.1111/iej.12592
Pankajakshan D, Voytik-Harbin SL, Nör JE, Bottino MC. Injectable highly tunable oligomeric collagen matrices for dental tissue regeneration. ACS Appl Bio Mater 2020;3:859–68. https://doi.org/10.1021/acsabm.9b00944
Chrepa V, Austah O, Diogenes A. Evaluation of a commercially available hyaluronic acid hydrogel (restylane) as injectable scaffold for dental pulp regeneration: an in vitro evaluation. J Endod. 2017;43:257–62. https://doi.org/10.1016/j.joen.2016.10.026
Yu H, Zhang X, Song W, Pan T, Wang H, Ning T, et al. Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells. J Endod. 2019;45:706–15. https://doi.org/10.1016/j.joen.2019.03.004
Bertassoni LE. Progress and challenges in microengineering the dental pulp vascular microenvironment. J Endod. 2020;46:S90–100. https://doi.org/10.1016/j.joen.2020.06.033
Monteiro N, Thrivikraman G, Athirasala A, Tahayeri A, França CM, Ferracane JL, et al. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent Mater. 2018;34:389–99. https://doi.org/10.1016/j.dental.2017.11.020
Luzuriaga J, Irurzun J, Irastorza I, Unda F, Ibarretxe G, Pineda JR. Vasculogenesis from human dental pulp stem cells grown in matrigel with fully defined serum-free culture media. Biomedicines. 2020;8:483 https://doi.org/10.3390/biomedicines8110483
Hu L, Gao Z, Xu J, Zhu Z, Fan Z, Zhang C, et al. Decellularized swine dental pulp as a bioscaffold for pulp regeneration. BioMed Res Int. 2017;2017:1–9. https://doi.org/10.1155/2017/9342714
Matoug-Elwerfelli M, Duggal MS, Nazzal H, Esteves F, Raïf E. A biocompatible decellularized pulp scaffold for regenerative endodontics. Int Endod J. 2018;51:663–73. https://doi.org/10.1111/iej.12882
Ravindran S, Huang CC, George A. Extracellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSCs. Front Physiol. 2014;4. https://doi.org/10.3389/fphys.2013.00395
Meng H, Hu L, Zhou Y, Ge Z, Wang H, Wu CT, et al. A sandwich structure of human dental pulp stem cell sheet, treated dentin matrix, and matrigel for tooth root regeneration. Stem Cells Dev. 2020;29:521–32. https://doi.org/10.1089/scd.2019.0162
Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgley C, et al. A novel strategy to engineer pre-vascularized full-length dental pulp-like tissue constructs. Sci Rep. 2017;7:3323. https://doi.org/10.1038/s41598-017-02532-3
Aksel H, Huang GTJ. Combined effects of vascular endothelial growth factor and bone morphogenetic protein 2 on odonto/osteogenic differentiation of human dental pulp stem cells in vitro. J Endod. 2017;43:930–5. https://doi.org/10.1016/j.joen.2017.01.036
He H, Yu J, Liu Y, Lu S, Liu H, Shi J, et al. Effects of FGF2 and TGFβ1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int. 2008;32:827–34. https://doi.org/10.1016/j.cellbi.2008.03.013
Xu F, Qiao L, Zhao Y, Chen W, Hong S, Pan J, et al. The potential application of concentrated growth factor in pulp regeneration: an in vitro and in vivo study. Stem Cell Res Ther. 2019;10:134. https://doi.org/10.1186/s13287-019-1247-4
Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP- 2 and odontoblast differentiation. J Dent Res. 2010;89:603–8. https://doi.org/10.1177/0022034510364487
Sadaghiani L, Gleeson HB, Youde S, Waddington RJ, Lynch CD, Sloan AJ. Growth factor liberation and dpsc response following dentine conditioning. J Dent Res. 2016;95:1298–307. https://doi.org/10.1177/0022034516653568
Elseed MA, Murray PE, Garcia-Godoy F, Namerow KN. Assessment of bioactive and bio-adhesive therapies to enhance stem cell attachment to root surface dentine. Int Endod J 2009;42:576–83. https://doi.org/10.1111/j.1365-2591.2009.01551.x
Melling GE, Colombo JS, Avery SJ, Ayre WN, Evans SL, Waddington RJ, et al. Liposomal delivery of demineralized dentin matrix for dental tissue regeneration. Tissue Eng Part A. 2018;24:1057–65. https://doi.org/10.1089/ten.tea.2017.0419
Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer PC, et al. EDTA conditioning of dentine promotes adhesion, migration, and differentiation of dental pulp stem cells. Int Endod J. 2016;49:581–90. https://doi.org/10.1111/iej.12492
Atesci AA, Avci CB, Tuglu MI, Ozates Ay NP, Eronat AC. Effect of different dentin conditioning agents on growth factor release, mesenchymal stem cell attachment and morphology. J Endod. 2020;46:200–8. https://doi.org/10.1016/j.joen.2019.10.033
Jin R, Song G, Chai J, Gou X, Yuan G, Chen Z. Effects of concentrated growth factor on proliferation, migration, and differentiation of human dental pulp stem cells in vitro. J Tissue Eng. 2018;9:204173141881750 https://doi.org/10.1177/2041731418817505
Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–15. https://doi.org/10.1016/j.joen.2011.05.013
Gruber-Bzura BM. [Cytotoxicity in vitro as the principal parameter for evaluation of biocompatibility of medical devices]. Wiad Lek. 2017;70:977–81.
Hanks CT, Craig RG, Diehl ML, Pashley DH. Cytotoxicity of dental composites and other materials in a new in vitro device. J Oral Pathol Med. 1988;17:396–403. https://doi.org/10.1111/j.1600-0714.1988.tb01304.x
Hanks CT, Diehl ML, Craig RG, Makinen PK, Pashley DH. Characterization of the “in vitro pulp chamber” using the cytotoxicity of phenol. J Oral Pathol Med. 1989;18:97–107. https://doi.org/10.1111/j.1600-0714.1989.tb00744.x
Schmalz G, Schuster U, Nuetzel K, Schweikl H. An in vitro pulp chamber with three-dimensional cell cultures. J Endod. 1999;25:24–29. https://doi.org/10.1016/S0099-2399(99)80394-X
França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, et al. The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip. 2020;20:405–13. https://doi.org/10.1039/C9LC00915A
Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, et al. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8:2135–57. https://doi.org/10.1038/nprot.2013.137
Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 2018;33:43–48. https://doi.org/10.1016/j.dmpk.2017.11.003
Dusinska M, Rundén-Pran E, Schnekenburger J, Kanno J. Toxicity Tests: In vitro and in vivo. In: Adverse Effects of Engineered Nanomaterials. Elsevier; 2017:51-82. https://doi.org/10.1016/B978-0-12-809199-9.00003-373.
Pedano MS, Li X, Camargo B, Hauben E, De Vleeschauwer S, Yoshihara K, et al. Injectable phosphopullulan-functionalized calcium-silicate cement for pulp-tissue engineering: An in-vivo and ex-vivo study. Dent Mater. 2020;36:512–26. https://doi.org/10.1016/j.dental.2020.01.011
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect Tissue Res. 2002;43:387–90. https://doi.org/10.1080/03008200290000574
Heywood BR, Appleton J. The ultrastructure of the rat incisor odontoblast in organ culture. Arch Oral Biol. 1984;29:327–9. https://doi.org/10.1016/0003-9969(84)90107-9
Tjäderhane L, Salo T, Larjava H, Larmas M, Overall CM. A novel organ culture method to study the function of human odontoblasts in vitro: gelatinase expression by odontoblasts is differentially regulated by TGF-β1. J Dent Res. 1998;77:1486–96. https://doi.org/10.1177/00220345980770070301
Hasegawa N. Effects of various culture conditions on matrix formative functions of rat incisor odontoblasts in a pulp-dentin slice culture system. Jpn J Oral Biol. 1989;31:392–403. https://doi.org/10.2330/joralbiosci1965.31.392
Sloan AJ, Shelton RM, Hann AC, Moxham BJ, Smith AJ. An in vitro approach for the study of dentinogenesis by organ culture of the dentine–pulp complex from rat incisor teeth. Arch Oral Biol. 1998;43:421–30. https://doi.org/10.1016/S0003-9969(98)00029-6
Magloire H, Joffre A, Bleicher F. An in vitro model of human dental pulp repair. J Dent Res. 1996;75:1971–8. https://doi.org/10.1177/00220345960750120901
Murray PE, Smith AJ, Garcia-Godoy F, Lumley PJ. Comparison of operative procedure variables on pulpal viability in an ex vivo model. Int Endod J. 2008;41:389–400. https://doi.org/10.1111/j.1365-2591.2007.01364.x
Téclès O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005;50:103–8. https://doi.org/10.1016/j.archoralbio.2004.11.009
Pedano MS, Li X, Jeanneau C, et al. Survival of human dental pulp cells after 4-week culture in human tooth model. J Dent. 2019;86:33–40. https://doi.org/10.1016/j.jdent.2019.05.023
Zaky SH, AlQahtani Q, Chen J, Patil A, Taboas J, Beniash E, et al. Effect of the periapical “inflammatory plug” on dental pulp regeneration: a histologic in vivo study. J Endod. 2020;46:51–56. https://doi.org/10.1016/j.joen.2019.10.006
Poimenova A, Kitraki E, Kakaboura A, Rahiotis C. Early responses of human pulp to direct capping with resin adhesive systems and calcium hydroxide. Dent Mater. 2018;34:e73–82. https://doi.org/10.1016/j.dental.2018.01.018
Saw TY, Cao T, Yap AUJ, Lee Ng MM. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol Vitr. 2005;19:145–54. https://doi.org/10.1016/j.tiv.2004.08.006
Seang S, Pavasant P, Limjeerajarus CN. Iloprost induces dental pulp angiogenesis in a growth factor–free 3-dimensional organ culture system. J Endod. 2018;44:759–64.e2. https://doi.org/10.1016/j.joen.2018.02.001
Chakka LRJ, Vislisel J, Vidal C, de MP, Biz MT K, Salem A, et al. Application of BMP-2/FGF-2 gene–activated scaffolds for dental pulp capping. Clin Oral Invest. 2020;24:4427–37. https://doi.org/10.1007/s00784-020-03308-2
Faggion CM. Animal research as a basis for clinical trials. Eur J Oral Sci. 2015;123:61–64. https://doi.org/10.1111/eos.12175
Moreira MS, Diniz IM, Rodrigues MF, de Carvalho RA, de Almeida Carrer FC, Neves II, et al. In vivo experimental model of orthotopic dental pulp regeneration under the influence of photobiomodulation therapy. J Photochem Photobio B: Biol. 2017;166:180–6. https://doi.org/10.1016/j.jphotobiol.2016.11.022
El-Backly RM, Massoud AG, El-Badry AM, Sherif RA, Marei MK. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J 2008;34:52–67.
Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421–6. https://doi.org/10.1016/j.joen.2008.02.005
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9. https://doi.org/10.1016/j.joen.2008.04.009
Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP- 2 and odontoblast differentiation. J Dent Res. 2010;89:603–8. https://doi.org/10.1177/0022034510364487
Demarco FF, Casagrande L, Zhang Z, Dong Z, Tarquinio SB, Zeitlin BD, et al. Effects of morphogen and scaffold porogen on the differentiation of dental pulp stem cells. J Endod. 2010;36:1805–11. https://doi.org/10.1016/j.joen.2010.08.031
Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012;33:2109–18.
Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A, et al. GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration. J Dent Res. 2017;96:192–9.
Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–6. https://doi.org/10.1177/0022034510368647
Alsanea R, Ravindran S, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. Biomimetic approach to perforation repair using dental pulp stem cells and dentin matrix protein 1. J Endod. 2011;37:1092–7. https://doi.org/10.1016/j.joen.2011.05.019
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–15. https://doi.org/10.1089/ten.tea.2009.0518
Syed-Picard FN, Ray HL, Kumta PN, Sfeir C. Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J Dent Res. 2014;93:250–5. https://doi.org/10.1177/0022034513517901
Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18:176–84. https://doi.org/10.1089/ten.tea.2011.0222
Galler KM, Brandl FP, Kirchhof S, Widbiller M, Eidt A, Buchalla W, et al. Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Eng Part A. 2018;24:234–44. https://doi.org/10.1089/ten.TEA.2016.0555
Widbiller M, Driesen RB, Eidt A, Lambrichts I, Hiller KA, Buchalla W, et al. Cell homing for pulp tissue engineering with endogenous dentin matrix proteins. J Endod. 2018;44:956–62.e2. https://doi.org/10.1016/j.joen.2018.02.011
Sequeira DB, Oliveira AR, Seabra CM, Palma PJ, Ramos C, Figueiredo MH, et al. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: in vivo interaction with two bioactive materials. Clin Oral Investig. 2021;25:5317–29. https://doi.org/10.1007/s00784-021-03840-9
Kodonas K, Gogos C, Papadimitriou S, Kouzi-Koliakou K, Tziafas D. Experimental formation of dentin-like structure in the root canal implant model using cryopreserved swine dental pulp progenitor cells. J Endod. 2012;38:913–9. https://doi.org/10.1016/j.joen.2012.02.005
Xu W, Jiang S, Chen Q, Ye Y, Chen J, Heng BC. Systemically transplanted bone marrow–derived cells contribute to dental pulp regeneration in a chimeric mouse model. J Endod. 2016;42:263–8.
Wang Y, Zhao Y, Jia W, Yang J, Ge L. Preliminary study on dental pulp stem cell– mediated pulp regeneration in canine immature permanent teeth. J Endod. 2013;39:195–201. https://doi.org/10.1016/j.joen.2012.10.002
Holland R, Sant’anna Júnior A, de Souza V. Influence of apical patency and filling material on healing process of dogs’ teeth with vital pulp after root canal therapy. Braz Dent J. 2005;16:9–16. https://doi.org/10.1590/S0103-64402005000100002
Iohara K, Murakami M, Takeuchi N, et al. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. STEM CELLS Transl Med. 2013;2:521–33. https://doi.org/10.5966/sctm.2012-0132
Palma PJ, Ramos JC, Martins JB, Diogenes A, Figueiredo MH, Ferreira P, et al. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J Endod. 2017;43:1279–87. https://doi.org/10.1016/j.joen.2017.03.005
Thibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680–9.
Zaky SH, Shehabeldin M, Ray H, Sfeir C. The role of inflammation modulation in dental pulp regeneration. Eur Cell Mater. 2021;41:184–93. https://doi.org/10.22203/eCM.v041a13.
Mangione F, EzEldeen M, Bardet C, Lesieur J, Bonneau M, Decup F. Implanted dental pulp cells fail to induce regeneration in partial pulpotomies. J Dent Res. 2017;96:1406–13.
Jang JH, Moon JH, Kim SG, Kim SY. Pulp regeneration with hemostatic matrices as a scaffold in an immature tooth minipig model. Sci Rep. 2020;10:12536 https://doi.org/10.1038/s41598-020-69437-6. Published 2020 Jul 27
Torabinejad M, Corr R, Buhrley M, Wright K, Shabahang S. An animal model to study regenerative endodontics. J Endod. 2011;37:197–202. https://doi.org/10.1016/j.joen.2010.10.011
Altaii M, Broberg M, Cathro P, Richards L. Standardisation of sheep model for endodontic regeneration/revitalization research. Arch Oral Biol. 2016;65:87–94. https://doi.org/10.1016/j.archoralbio.2016.01.008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piglionico, S.S., Pons, C., Romieu, O. et al. In vitro, ex vivo, and in vivo models for dental pulp regeneration. J Mater Sci: Mater Med 34, 15 (2023). https://doi.org/10.1007/s10856-023-06718-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-023-06718-2