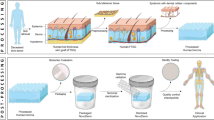
Abstract
Detergent treatment is the most commonly used method for the decellularization of ligaments and tendon grafts. However, it is well recognized that detergent treatment can also adversely affect the extracellular matrix. This study found that discission into the aponeurosis layer of the patellar tendon (PT) before decellularization is conducive to extracting cells from the PT using a low quantity of detergent in a short time period. The acellular aponeurosis discission ligament (AADL) retains its native collagen fibril structure and mechanical properties. Moreover, the PT retained cell and tissue compatibility in vitro and in vivo. After implantation into a defective allogeneic PT, we found that the AADL healed well in the host, and its collagen structure exhibited gradual improvement 12 months after implantation with satisfactory reconstruction.
Impact
The aponeurosis of tendons/ligaments is the main barrier to achieving complete decellularization, and it thus prevents complete recellularization for applications in tissue engineering. Aponeurosis can obstruct the removal of cell components. We found that excising the aponeurosis before decellularization allows for the removal of cellular components with a reduced amount of detergent, thus improving the biological properties of the acellular ligament. To the best of our knowledge, no similar studies have been performed.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Current tissue-engineering ligament scaffolds are derived from a variety of substances, such as silk [1], natural collagen [2], polyglycolic acid/polylactic acid and their copolymer (PLGA/PLLA) fibers [3,4,5], and ligament/tendon extracellular matrices [6,7,8,9]. Acellular ligament scaffolds have the properties of the native ligament extracellular matrix (ECM). Its immunogenicity is reduced by the extraction of cell components [10,11,12,13] and has only minimal antigenicity preserved due to type-I collagen [14, 15]. Therefore, acellular ligament scaffolds are expected to be the ideal tissue-engineered ligament scaffold.
Existing acellular ligament scaffold studies utilize pig anterior cruciate ligaments [6], pig or rabbit patellar tendons [7, 8], and chicken toe deep tendons [9]. Decellularization protocols are often combined with physical, enzymatic, and chemical treatments. The application of chemical detergent is the primary step to achieving decellularization [16]. The physical and enzymatic decellularization of ligaments/tendons do not differ much; however, detergents used for their decellularization effects have been reported to exhibit different properties in different species, tissues, and ligament/tendon sizes [6,7,8,9,10, 16,17,18]. Commonly used detergents for ligament decellularization contain sodium dodecyl sulfate, tri (n-butyl) phosphate, and Triton X-100, all of which may cause damage to collagen and the glycosaminoglycans of the ECM [6, 17]. Therefore, reducing the dose of the detergent may protect the biological characteristics of the ligament ECM.
In our previous study on the decellularization of the rabbit Achilles tendon, we found that, when treated with hypotonic Tris buffer for 48 h in a 150-rpm shaker without chemical detergents, a small quantity of residual cells accumulated under the aponeurosis deep in the tendon. However, in the part of the tendon without an intact, covered aponeurosis, cells were completely removed as determined by hematoxylin and eosin (H&E) staining (Fig. 1). This indicates that the aponeurosis could obstruct the removal of cell components. Therefore, we expected that excising the aponeurosis before decellularization would help to remove cellular components with a reduced amount of detergent, thus improving the biological properties of the acellular ligament.
Hematoxylin and eosin (H&E) staining of the rabbit Achilles tendon treated with hypotonic Tris buffer without chemical detergents. Major nuclear components accumulated under the intact aponeurosis (black arrow), and a small amount of residual nuclear components were visible at the edge of the incomplete aponeurosis (white arrow) (magnification ×100)
2 Materials and methods
2.1 Harvest of patella tendons
The patella tendons (PT) of New Zealand white rabbits (2.5–3.0 kg, 6-month-old male rabbits) obtained from the Second Military Medical University Experimental Animal Center in China were used in this study. The animal husbandry, experiments, and sacrifice were performed according to the animal ethics standards. Induced euthanasia was applied by slow injection of excessive sodium pentobarbital. The patella-PT (12 mm × 8 mm × 1 mm)-tibia complexes (Fig. 2a) were harvested using aseptic techniques. The synovial soft tissue around them was cleared without disrupting the integrity of the PT aponeurosis and then placed into sterile phosphate buffered saline (PBS) for future use. All procedures complied with the ethical use protocols of the Second Military Medical University Experimental Animal Center.
2.2 Preparation of PT aponeuroses prior to decellularization
The aponeuroses of group 1 were excised on both sides of the PT to expose the inner collagen fibers (Fig. 2b). The specimens of group 2 contained intact PT aponeurosis prior to decellularization, and those of group 3 contained fresh PT (FPT) and were used as the control group.
2.3 Decellularization method
The decellularization steps performed were as follows: specimens were (1) immersed into a 10-mM hypotonic Tris buffer for 24 h, (2) transferred to a 50-mM Tris buffer containing a high concentration of saline (1.5 M KCl) for 24 h, (3) treated with 0.05% trypsin for 5 h, (4) washed with PBS and 1% Triton X-100 for 48 h, and (5) washed with PBS for 72 h with the PBS changed every 24 h. All decellularization steps were performed with a serine protease inhibitor (5% phenylmethylsulfonylfluoride in PBS, 0.35 ml/l), a metalloprotease inhibitor (5 mM ethylenediaminetetraacetic acid (EDTA)) and 5 ml/l penicillin solution (10,000 U/ml/10,000 mg/ml) at 37 °C with shaking (100 rpm).
2.4 Histology and scanning electron microscopy analysis
Mid-substance portions of the specimens were obtained and placed in 10% formalin for 8 h at room temperature, dehydrated, embedded in paraffin, microtomed into 5.0-μm thick slices (longitudinal and cross sections), mounted onto slides, and stained with H&E (Sigma). To analyze the nuclear components and collagen structure, a microscope was used to image the sections (NIKON E-600).
After washing with double distilled water, the 10% formalin-fixed specimens were freeze-dried (VirTis BENCHTOP), cut into 1 mm × 1 mm × 1 mm sections, sprayed with a conductive coating to coat the surface, and scanned on an electron microscope (JSM-6700F) [19]. Images were examined for residual cell debris and microstructural changes in the collagen fibrils.
2.5 Determination of DNA and collagen content
Freeze-dried specimens from groups 1, 2, and 3 (dry weight of 60 mg) were recorded. DNA contents were extracted using a tissue genomic DNA extraction kit (TIANamp Genomic DNA Kit; TIANGEN Co., Germany). The DNA concentration in the resulting preparation was used to calculate the total DNA content at an absorbance of 280 nm using a spectrophotometer (Thermo Spectronic, VARIOSKAN) and was then normalized to the initial dry weight of the sample.
Hydroxyproline contents were estimated using a hydroxyproline test kit (Nanjing Jiancheng Bioengineering Institute, China) and measured at an absorbance of 557 nm using a spectrophotometer (Thermo Spectronic, VARIOSKAN). The amount of collagen per dry weight of the sample was determined assuming 13.4% hydroxyproline content in collagen.
2.6 Mechanical testing
Since the maximum load of a complete rabbit PT is more than 300 N and patella fracture often occurs prior to ligament rupture during mechanical tests, we cut the force area of specimens into 3-mm wide sections (Fig. 3) and used a maximum load of ~200 N to obtain effective biomechanics data of PTs. This load would not lead to patella fracture before ligament rupture. All specimens were stored in PBS at room temperature for 4 h before testing. Their ends, the patella and tibia, were wrapped with PBS-soaked gauze and placed into the grips of a uniaxial load frame (Instron 5542, Needham, MA) to test the maximum load and elastic modulus. Specimens were preloaded at 1 N and loaded at a rate of 15 mm/min, and all PTs were moist with PBS during testing. The measured data for ligament force area rupture without fracture of the patella or tibia were then validated.
2.7 In vitro cytocompatibility of the acellular aponeurosis-discission ligament (AADL)
2.7.1 Coculture of cells and the AADL
We cut 50-μm thick slices of AADL with a frozen microtome (LEICA) and washed and preserved them with sterile PBS; further, we packaged these into 15 ml sterile centrifuge tubes and then disinfected them with γ-rays (16 K Gray). The sterile slices were cut into ~10 mm × 10 mm sections and transferred to individual wells (n = 6) of a 96-well plate. The wells were coated with collagen (from calf skin, C8919, 10 μg/cm2; Sigma) before use and sterilized by overnight exposure to UV light. Furthermore, 100 μl of rabbit PT fibroblast suspension (1 × 104 cells, passage 3, in 10% FBS-DMEM media) tagged with green fluorescent protein (GFP) was dropped onto the surface of the AADL slices and collagen-coated wells and incubated at 37 °C with 5% CO2 for 72 h.
2.7.2 Colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell proliferation was measured by the MTT dye-reduction assay. Briefly, cells were incubated for 24, 48, and 72 h, and coculture specimens were incubated for 4 h at 37 °C with 10 μl MTT solution (5 mg/ml). After removal of the media containing MTT, 100 μl dimethyl sulfoxide was added, and the plates were shaken at room temperature for 10 min. The absorbance was measured at 540 nm using a 96-well plate spectrophotometer.
2.7.3 Observation of cells on the AADL
After 24 and 72 h, cocultures and fibroblasts were directly observed on the AADL surface using a fluorescence microscope (NIKON E-600).
At 72 h, the cocultures were fixed with 4% paraformaldehyde for 4 h, rinsed with distilled water, and then lyophilized for electron microscopy examination.
2.8 In vivo cytocompatibility and histocompatibility of the AADL
The center of the PT of New Zealand white rabbits, which was ~3 mm × 10 mm × 10 mm in size, was incised to create a defect. The γ-ray (16 K Gray)-disinfected AADL was then implanted and fixed into the PT defect by using a 3–0 nylon suture line for surgery (Fig. 4). Specimens were harvested at 3 weeks, 6 months, and 12 months after implantation and grossly and histologically examined for signs of healing, inflammation/immune response, host cell infiltration, and modification of the AADL structure.
2.9 Statistical analysis
The data from the DNA content, collagen content, tensile analyses, and MTT assay are expressed as the mean values ± standard deviation. The data were subjected to one-way analysis of variance. Statistical significance was determined by an LSD t-test, where p values ≤ 0.05 were considered significant.
3 Results
3.1 Histological examination of decellularization
No nuclear components were detected within the AADL; the collagen structure arrangement did not obviously change (Fig. 5b) compared with that of the normal PT (Fig. 5a), but the interspaces between the collagen fibers were increased after decellularization. In the matrix with the intact aponeurosis treated by the same decellularization procedures, more residual nuclear components accumulated under the aponeurosis (Fig. 5c), as shown by H&E staining. Thus, using the same decellularization procedure, cells in the PT that were removed with aponeurosis discission were more vulnerable than those with an intact aponeurosis.
Hematoxylin and eosin (H&E) staining of specimens after decellularization. a1, a2 Longitudinal sections and cross sections of patella tendons (PT); b1, b2 longitudinal sections and cross sections of acellular aponeurosis-discission ligament (AADL); c1, c2 longitudinal sections and cross sections of an intact aponeurosis after decellularization. (magnification ×200)
3.2 Scanning electron microscopy analysis of decellularization
Electron microscopy examination showed cell debris in freeze-dried normal PT (Fig. 6a); no significant residual cell debris was observed in the AADL, but the interspaces between the collagen fibers had significantly increased (Fig. 6b). The AADL collagen fibril structure (Fig. 6d) showed no change compared to the normal PT (Fig. 6c).
Scanning electron microscopy of specimens after decellularization. a Cell debris (white arrow) seen on the cross section of normal freeze-dried patella tendons (PT) (magnification ×300); b no cell debris are seen on the acellular aponeurosis-discission ligament (AADL), but the interspaces between the collagen fibers are increased; c the normal PT collagen fibrils exhibit a spiral shape (magnification ×20,000); d the AADL collagen fibril structure shows no change compared to that of the normal PT (magnification ×30,000)
3.3 Determination of DNA content and collagen content
The results of DNA content and collagen content tests are shown in Fig. 7. The DNA content of AADL (0.0415 ± 0.005 μg/mg) was significantly lower than that of the natural PT (p < 0.05), but that of group 2 (0.1632 ± 0.0243 μg/mg) showed no significant difference compared to the natural PT (0.1977 ± 0.015 μg/mg, p > 0.05). The collagen content in specimens of group 1 (822.76 ± 61.26 μg/mg), group 2 (803.90 ± 37.93 μg/mg), and group 3 (825.51 ± 44.43 μg/mg) showed no significant difference (Fig. 7, p > 0.05).
3.4 Mechanical testing
Mechanical testing of the AADL showed that the maximum load at rupture was 214.33 ± 7.26 N (n = 6), and a slight decrease was noted compared to the maximum FPT load, which was 222.17 ± 12.42 N (n = 6), but the difference was not statistically significant (p > 0.05). No significant difference was observed with regard to the elastic modulus between AADL (13.01 ± 1.84 MPa) and FPT (13.24 ± 2.18 MPa) (Fig. 8, p > 0.05).
3.5 In vitro cytocompatibility of the AADL
The colorimetric MTT assays showed that the absorbance at 540 nm of cells on AADL slices was slightly lower than that on collagen coating, but this had no significant effect on the proliferation of cells on either surface (p > 0.05) (Fig. 9).
After 24 h of coculturing (Fig. 10a), GFP-tagged fibroblasts were directly observed on AADL slices by fluorescence microscopy. The cells were isolated, and they exhibited longitudinally oriented collagen fibers; moreover, the cell density did not change significantly at 72 h (Fig. 10b). Scanning electron microscopy analysis showed that cells were attached to the AADL collagen fibers (Fig. 10d).
Fluorescence microscopy and scanning electron microscopy of GFP-tagged fibroblasts on specimens. a Cocultures at 24 h show more fibroblasts attached to the acellular aponeurosis-discission ligament (AADL) surface (as indicated by the white arrow on the green fluorescent display, magnification ×400); b the fibroblast density in cocultures incubated for 72 h did not change significantly (magnification ×400); c AADL surface before coculture with cells (magnification ×300); d cocultures at 72 h show more seeded cells (indicated by the white arrow) attached to the AADL surface (magnification ×335)
3.6 In vivo host cytocompatibility and histocompatibility
From qualitative observation, the rabbits’ wounded knee joints showed good activity; the implants healed well with their surrounding ligaments, and no severe adhesion or significant formation of granulation was found around the AADL after implantation at 3 weeks, 6 months, or 12 months (Fig. 11a). At 3 weeks, the site showed that fibroblast-like cells migrated deep into the AADL and oriented longitudinally along the long axis of the collagen fibers. However, a small number of monocytes were distributed at their interface (Fig. 11c 3 W), and their collagen fibers appeared slightly looser than those of the host PT (Fig. 11b 3 W). At 6 months, the structure of the AADL collagen fibers improved, and the amount of monocyte infiltration decreased (Fig. 11b and 11 6 M). At 12 months, the AADL integrated well with the host PT, whose fibroblast-like cell distribution and collagen fiber structure resembled that of the host PT. In addition, more angiogenesis was found at the healing interface (Fig. 11b and 11 12 M). No significant evidence of infiltration by inflammatory cells, such as multinuclear giant cells, eosinophils, lymphocytes, or macrophages, was observed at any time point.
In vivo host cytocompatibility and histocompatibility results. a Gross observation at 3 weeks, 6 months, and 12 months after implantation. The implants are indicated by black arrows. b, c Longitudinal section and cross section of hematoxylin and eosin (H&E) staining (magnification ×200). The right area of the dashed line indicates the AADL, whereas the left area is the host PT. A small monocyte distribution is indicated by the white arrow (c 3 W), and angiogenesis is also indicated by the white arrow (b 12 M and c 12 M; magnification ×100)
4 Discussion
Most of the current strategies to construct tissue-engineered ligaments have relied on cells seeded into a scaffold. Although the acellular ligament scaffold showed characteristics that were similar to its natural ECM, its porosity was not suitable for seeding cells deep into the tissue in vitro [8, 20, 21]. However, this did not obstruct the migration of fibroblast-like host cells deep into the acellular ligament scaffold in vivo [9]. If the fibroblast-like host cells and other relevant host cells can remold the acellular ligament scaffold with sufficient biomechanical integrity to withstand rehabilitation, the steps of generating and seeding cells into the scaffold may be omitted. Therefore, it is of great interest to identify a method to construct an acellular scaffold that preserves the biological properties of the ECM of the ligament.
The ideal method for the decellularization of ligaments would completely extract the cells while retaining their native structure and biological properties. The study presented here demonstrates that cells are more vulnerable to removal in aponeurosis-discission PT than in tissue that has an intact aponeurosis using the same procedures for decellularization. The dosage of chemical detergents and the decellularization procedure were also reduced compared with those reported by Cartmell [7]. No obvious residual cellular components of the AADL were identified by histological and scanning electron microscopic analyses. Its mean residual DNA content (0.04 μg/mg) was lower than that in commercially available acellular scaffolds, such as Restore™ (Porcine small intestinal submucosa) and TissueMend™ (Bovine fetal dermis) [22], as well as in the acellular ligament scaffold reported by Whitlock [9]. The AADL also had satisfactory mechanical properties and good cytocompatibility in vitro, likely due to its retention of the native ultrastructure of collagen fibrils. The AADL also proved to integrate well with the host ligament in vivo. Histological examination showed that host fibroblast-like cells that migrated deep into the AADL were distributed evenly and oriented longitudinally along the long axis of its collagen fibers. The structure of the AADL collagen fibers was similar to that of the host ligament. In addition, no obvious immune rejection or inflammatory responses were induced by the host PT.
During the decellularization processes, the internal collagen fibers of the aponeurosis-discission ligament are more exposed to the decellularized reagents. Therefore, we should select reagents that have little negative effect on collagen protein. The main function of Triton X-100 is to disrupt lipid–lipid and lipid–protein interactions while leaving protein–protein interactions intact; the functions of the low concentrations of trypsin and EDTA are mainly to disrupt cell adhesion to the ECM, and DNase is used to hydrolyze the DNA components [16]. Therefore, these selected reagents should have no negative effect on the collagen protein [23]. Although the interspaces between collagen fibers in the AADL were increased, no significant changes in its mechanical properties were observed. This is likely due to the continuous structure of the mature ligament/tendon fiber and the fact that force is directly transmitted through the collagen fibers rather than the connection collagen structure [24,25,26,27,28].
Allogeneic/xenograft antigenicity is mainly conferred by the Gal antigen in the cell membrane, DNA, and related proteins [10,11,12,13, 29]. The antigenicity of an acellular ligament matrix is primarily due to being largely made up of collagen. Because the collagen structure is conserved across species [30,31,32], it possesses little inherent immunogenic potential [15, 29]. When used as tissue engineering scaffolds, seeded cells did not easily reseed into the interior of the acellular matrix ligament as determined from in vitro tests [8, 9, 21, 28, 32], but, in vivo, host fibroblast-like cells were still able to migrate into the AADL. In this study, an histological examination revealed that the collagen structure of the acellular ligament matrix gradually improved at 3 weeks, 6 months, and 12 months after implantation. Whether acellular matrix remodeling was related to the migration of fibroblast-like cells into the tissue and how these affect reconstruction and biomechanical changes remains unclear and is an active area of research.
5 Summary
Intact aponeurosis of the ligament can hinder the removal of cells during decellularization processes. Using an appropriate aponeurosis discission before decellularization, cell components were extracted more easily with a low dose of detergent in a short time period. This decellularization method showed no obvious impact on collagen fibril structure and no significant adverse effect on mechanical properties. The AADL also exhibited good cytocompatibility and histocompatibility, and the results of the H&E staining revealed that the allogeneic PT defects reconstructed by AADL at 12 months after implantation were satisfactory.
References
Sun W, Gregory DA, Tomeh MA, Zhao X. Silk fibroin as a functional biomaterial for tissue engineering. Int J Mol Sci. 2021;22:1499.
Butler DL, Gooch C, Kinneberg KR. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat Protoc. 2010;5:849–63.
Vaquette C, Kahn C, Frochot C. Aligned poly(L-lactic-co-e-caprolactone) electrospun microfibers and knitted structure: a novel composite scaffold for ligament tissue engineering. J Biomed Mater Res A. 2010;94:1270–82.
Sahoo S, Toh SL, Goh JC. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990–8.
Huebner P, Warren PB, Chester D, Spang JT, Brown AC, Fisher MB, et al. Mechanical properties of tissue formed in vivo are affected by 3D-bioplotted scaffold microarchitecture and correlate with ECM collagen fiber alignment. Connect Tissue Res. 2020;61:190–204.
Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone–anterior cruciate ligament–bone graft. Biomaterials. 2005;26:7339–49.
Cartmell JS, Dunn MG. Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng. 2004;10:1065–75.
Ingram JH, Korossis S, Howling G, Fisher J, Ingham E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007;13:1561–72.
Whitlocka PW, Smitha TL, Poehlinga GG, Shilta JS, Dykeb MV. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials. 2007;28:4321–9.
Collins BH, Chari RS, Magee JC, Harland RC, Lindman BJ, Logan JS, et al. Mechanisms of injury in porcine livers perfused with blood of patients with fulminant hepatic failure. Transplantation. 1994;58:1162–71.
Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205.
Galili U, Macher BA, Buehler J, Shohet SB. Human natural anti-alphagalactosyl IgG. II. The specific recognition of alpha (1–3)-linked galactose residues. J Exp Med. 1985;162:573–82.
Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–42.
Doroski DM, Brink KS, Temenoff JS. Techniques for biological characterization of tissue-engineered tendon and ligament. Biomaterials. 2007;28:187–202.
Lynn AK, Yannas BW IV. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–54.
Gilbert TW, Sellaro TL, Badylaka SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83.
Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–40.
Harrison RD, Gratzer PF. Effect of extraction protocols and epidermal growth factor on the cellular repopulation of decellularized anterior cruciate ligament allografts. J Biomed Mater Res A. 2005;75:841–54.
Zhou S, Yuan B, Huang W, Tang Y, Chen X. Preparation and biological characteristics of a bovine acellular tendon fiber material. J Biomed Mater Res A. 2021;109:1931–41.
Gratzer PF, Harrison RD, Woods T. Matrix alteration and not residual sodium sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006;12:2975–83.
Tischer T, Vogt S, Aryee S, Steinhauser E, Adamczyk C, Milz S. Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Arch Orthop Trauma Surg. 2007;127:735–41.
Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone J Surg. 2006;88:2665–72.
Raghavan SS, Woon CY, Kraus A, Megerle K, Choi MS, Pridgen BC, et al. Human flexor tendon tissue engineering: decellularization of human flexor tendons reduces immunogenicity in vivo. Tissue Eng Part A. 2012;18:796–805.
Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–53.
Williams LN, Elder SH, Horstemeyer MF, Harbarger D. Variation of diameter distribution, number density, and area fraction of fibrils within fiveareas of the rabbit patellar tendon. Ann Anat. 2008;190:442–51.
Franchi M, Quaranta M, Macciocca M, De Pasquale V, Ottani V, Ruggeri A. Structure relates to elastic recoil and functional role in quadriceps tendon and PT. Micron. 2009;40:370–7.
Provenzano PP, Vanderby R,Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84.
Rothrauff BB, Lauro BB, Yang G, Debski RE, Musahl V, Tuan RS. Braided and stacked electrospun nanofibrous scaffolds for tendon and ligament tissue engineering. Tissue Eng Part A. 2017;23:378–89.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16.
Bernard MP, Myers JC, Chu ML, Prockop DJ. Structure of a cDNA for the pro- alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983;22:1139–45.
Kuivaniemi H, Tromp G, Chu ML, Prockop DJ. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988;252:633–40.
Ala-Kokko L, Kontusaari S, Baldwin CT, Kuivaniemi H, Prockop DJ. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989;260:509–16.
Acknowledgements
The authors gratefully acknowledge funding support from the National High-Tech Research and Development Program (863 Program, 2008AA02Z418) and the Science and Technology Commission of Shanghai Municipality (16XD1403400 and 201540190). The authors thank the Institute of Biomedical Engineering of the Second Military Medical University of China, Shanghai Key Laboratory of Tissue Engineering and Instrumental Analysis, and Research Center of Shanghai University for providing tissue and technical assistance. The authors would like to express their thanks to Professor Jianxin Dai of the Second Military Medical University of China for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Sy., Yuan, B., Huang, Wm. et al. Aponeurosis discission, a low-detergent method for tissue-engineered acellular ligament scaffolds. J Mater Sci: Mater Med 33, 40 (2022). https://doi.org/10.1007/s10856-022-06661-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-022-06661-8