Abstract
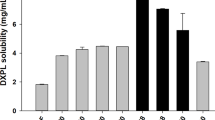
Curcumin (CUR) is a hydrophobic polyphenol with anti-inflammatory activity. However, its low water-solubility and poor skin permeation limited its application in the treatment of dermititis. CUR-loaded micelles were prepared using thin membrane hydration method with methoxy poly (ethylene glycol)-block-poly (ε-caprolactone) (MPEG-PCL) as carrier material. The drug loading capacity and encapsulation efficiency were 12.14 ± 0.33 and 93.57 ± 1.67%, respectively. CUR-loaded micelles increased CUR’s water-solubility to 1.87 mg/mL, being 1.87 × 106-folds higher than native CUR. CUR-loaded supramolecular hydrogels (CUR-H) were prepared through mixing the CUR-loaded micelles solution with α-cyclodextrin (α-CD) solution. The CUR-H presented continuous dissolution behaviour in aqueous medium for 4.5 h. The ex vivo skin permeation test and confocal fluorescence microscopy evaluation confirmed that CUR-H obviously enhanced skin deposition of CUR without drug flux from skin. In vivo experimental results confirmed that the CUR-H was more effective than dexamethasone ointments against croton oil-induced ear edema. The CUR-H composed of MPEG-PCL and α-CD is a promising formulation for skin inflammatory treatment.







Similar content being viewed by others
References
Chen Z, Xing L, Fan Q, Cheetham AG, Lin R, Holt B, et al. Drug-bearing supramolecular filament hydrogels as anti-inflammatory agents. Theranostics. 2017;7:2003–14. https://doi.org/10.7150/thno.19404
BB A, W Y, S L, SC G. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529–42. https://doi.org/10.1002/mnfr.201200838
Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, Chai KY, et al. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomed Pharmacother. 2008;62:630–6. https://doi.org/10.1016/j.biopha.2008.01.008
Panahi Y, Fazlolahzadeh O, Atkin SL, Majeed M, Butler AE, Johnston TP, et al. Evidence of curcumin and curcumin analogue effects in skin diseases: a narrative review. J Cell Physiol. 2018. https://doi.org/10.1002/jcp.27096.
Letchford K, Liggins R, Burt H. Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: theoretical and experimental data and correlations. J Pharm Sci. 2008;97:1179–90. https://doi.org/10.1002/jps.21037
Manju S, Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J Colloid Interface Sci. 2011;359:318–25. https://doi.org/10.1016/j.jcis.2011.03.071
Harrison IP, Spada F. Hydrogels for atopic dermatitis and wound management: a superior drug delivery vehicle. Pharmaceutics . 2018;10:71 https://doi.org/10.3390/pharmaceutics10020071
Li XY, Chen S, Zhang BJ, Li M, Diao K, Zhang ZL, et al. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int J Pharm. 2012;437:110–9. https://doi.org/10.1016/j.ijpharm.2012.08.001
Chen X, Zhi F, Jia XF, Zhang X, Ambardekar R, Meng ZJ, et al. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol. 2013;65:807–16. https://doi.org/10.1111/jphp.12043
Gong CY, Wu QJ, Wang YJ, Zhang DD, Luo F, Zhao X, et al. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013;34:6377–87. https://doi.org/10.1016/j.biomaterials.2013.05.005
Koop HS, de Freitas RA, de Souza MM, Savi-Jr R, Silveira JL. Topical curcumin-loaded hydrogels obtained using galactomannan from Schizolobium parahybae and xanthan. Carbohydr Polym. 2015;116:229–36. https://doi.org/10.1016/j.carbpol.2014.07.043
Sun YB, Du LN, Liu YP, Li X, Li M, Jin YG, et al. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-beta-cyclodextrin for melanoma treatment. Int J Pharm. 2014;469:31–9. https://doi.org/10.1016/j.ijpharm.2014.04.039
Nguyen HT, Munnier E, Souce M, Perse X, David S, Bonnier F, et al. Novel alginate-based nanocarriers as a strategy to include high concentrations of hydrophobic compounds in hydrogels for topical application. Nanotechnology. 2015;26:255101 https://doi.org/10.1088/0957-4484/26/25/255101
Bachhav YG, Mondon K, Kalia YN, Gurny R, Moller M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J Control Release. 2011;153:126–32. https://doi.org/10.1016/j.jconrel.2011.03.003
Poree DE, Giles MD, Lawson LB, He J, Grayson SM. Synthesis of amphiphilic star block copolymers and their evaluation as transdermal carriers. Biomacromolecules. 2011;12:898–906. https://doi.org/10.1021/bm101185t
Lapteva M, Mondon K, Moller M, Gurny R, Kalia YN. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis. Mol Pharm. 2014;11:2989–3001. https://doi.org/10.1021/mp400639e
Lapteva M, Santer V, Mondon K, Patmanidis I, Chiriano G, Scapozza L, et al. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J Control Release. 2014;196:9–18. https://doi.org/10.1016/j.jconrel.2014.09.021
Deng P, Teng F, Zhou F, Song Z, Meng N, Feng R. Methoxy poly (ethylene glycol)-b-poly (delta-valerolactone) copolymeric micelles for improved skin delivery of ketoconazole. J Biomater Sci Polym Ed. 2017;28:63–78. https://doi.org/10.1080/09205063.2016.1244371
Deng PZ, Teng FF, Zhou FL, Song ZM, Meng N, Liu N, et al. Y-shaped methoxy poly (ethylene glycol)-block-poly (epsilon-caprolactone)-based micelles for skin delivery of ketoconazole: in vitro study and in vivo evaluation. Mater Sci Eng C Mater Biol Appl. 2017;78:296–304. https://doi.org/10.1016/j.msec.2017.04.089
Zhao SP, Zhang LM, Ma D. Supramolecular hydrogels induced rapidly by inclusion complexation of poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) block copolymers with alpha-cyclodextrin in aqueous solutions. J Phys Chem B. 2006;110:12225–9. https://doi.org/10.1021/jp057506u
Varan C, Bilensoy E. Development of implantable hydroxypropyl-beta-cyclodextrin coated polycaprolactone nanoparticles for the controlled delivery of docetaxel to solid tumors. J Incl Phenom Macrocycl Chem. 2014;80:9–15. https://doi.org/10.1007/s10847-014-0422-6
Qiu L, Zhang L, Zheng C, Wang R. Improving physicochemical properties and doxorubicin cytotoxicity of novel polymeric micelles by poly(epsilon-caprolactone) segments. J Pharm Sci. 2011;100:2430–42. https://doi.org/10.1002/jps.22468
Khodaverdi E, Heidari Z, Tabassi SA, Tafaghodi M, Alibolandi M, Tekie FS, et al. Injectable supramolecular hydrogel from insulin-loaded triblock PCL-PEG-PCL copolymer and gamma-cyclodextrin with sustained-release property. AAPS PharmSciTech. 2015;16:140–9. https://doi.org/10.1208/s12249-014-0198-4
Payyappilly S, Dhara S, Chattopadhyay S. Thermoresponsive biodegradable PEG0-PCL-PEG based injectable hydrogel for pulsatile insulin delivery. J Biomed Mater Res Part A. 2014;102:1500–9. https://doi.org/10.1002/jbm.a.34800
Gong CY, Wu QJ, Dong PW, Shi SA, Fu SZ, Guo G, et al. Acute toxicity evaluation of biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PEG-PCL-PEG hydrogel. J Biomed Mater Res B. 2009;91b:26–36. https://doi.org/10.1002/jbm.b.31370
Gong CY, Shi SA, Dong PW, Yang B, Qi XR, Guo G, et al. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL-PEG-PCL hydrogel: part 1-synthesis, characterization, and acute toxicity evaluation. J Pharm Sci. 2009;98:4684–94. https://doi.org/10.1002/jps.21780
Fang F, Gong CY, Dong PW, Fu SZ, Gu YC, Guo G, et al. Acute toxicity evaluation of in situ gel-forming controlled drug delivery system based on biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) copolymer. Biomed Mater. 2009;4:025002 https://doi.org/10.1088/1748-6041/4/2/025002
Baek JS, Lim JH, Kang JS, Shin SC, Jung SH, Cho CW. Enhanced transdermal drug delivery of zaltoprofen using a novel formulation. Int J Pharm. 2013;453:358–62. https://doi.org/10.1016/j.ijpharm.2013.05.059
Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin-beta-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech. 2013;14:1303–12. https://doi.org/10.1208/s12249-013-0023-5
Lee H, Zeng F, Dunne M, Allen C. Methoxy poly(ethylene glycol)-block-poly(delta-valerolactone) copolymer micelles for formulation of hydrophobic drugs. Biomacromolecules. 2005;6:3119–28. https://doi.org/10.1021/bm050124+
Mathes C, Melero A, Conrad P, Vogt T, Rigo L, Selzer D, et al. Nanocarriers for optimizing the balance between interfollicular permeation and follicular uptake of topically applied clobetasol to minimize adverse effects. J Control Release. 2016;223:207–14. https://doi.org/10.1016/j.jconrel.2015.12.010
Gong C, Deng S, Wu Q, Xiang M, Wei X, Li L, et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials. 2013;34:1413–32. https://doi.org/10.1016/j.biomaterials.2012.10.068
Rehman K, Zulfakar MHJDD. Pharmacy I. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm. 2014;40:433–40. https://doi.org/10.3109/03639045.2013.828219
Che JX, Wu ZS, Shao WY, Guo PH, Lin YY, Pan WH, et al. Synergetic skin targeting effect of hydroxypropyl-beta-cyclodextrin combined with microemulsion for ketoconazole. Eur J Pharm Biopharm. 2015;93:136–48. https://doi.org/10.1016/j.ejpb.2015.03.028
Conte C, Costabile G, d’Angelo I, Pannico M, Musto P, Grassia G, et al. Skin transport of PEGylated poly(epsilon-caprolactone) nanoparticles assisted by (2-hydroxypropyl)-beta-cyclodextrin. J Colloid Interface Sci. 2015;454:112–20. https://doi.org/10.1016/j.jcis.2015.05.010
Gou ML, Men K, Shi HS, Xiang ML, Zhang J, Song J, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale . 2011;3:1558–67. https://doi.org/10.1039/c0nr00758g
Yallapu MM, Jaggi M, Chauhan SC. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79:113–25. https://doi.org/10.1016/j.colsurfb.2010.03.039
Ma MF, Sun T, Xing PY, Li ZL, Li SY, Su J, et al. A supramolecular curcumin vesicle and its application in controlling curcumin release. Colloid Surf A. 2014;459:157–65. https://doi.org/10.1016/j.colsurfa.2014.06.043
Grant N, Zhang HF. Poorly water-soluble drug nanoparticles via an emulsion-freeze-drying approach. J Colloid Interface Sci. 2011;356:573–8. https://doi.org/10.1016/j.jcis.2011.01.056
Ma D, Zhang LM, Xie X, Liu T, Xie MQ. Tunable supramolecular hydrogel for in situ encapsulation and sustained release of bioactive lysozyme. J Colloid Interface Sci. 2011;359:399–406. https://doi.org/10.1016/j.jcis.2011.04.032
Anitha A, Maya S, Deepa N, Chennazhi KP, Nair SV, Jayakumar R. Curcumin-Loaded N,O-Carboxymethyl chitosan nanoparticles for cancer drug delivery. J Biomater Sci Polym Ed. 2011;23:1381–400. https://doi.org/10.1163/092050611X581534
Zhu W, Li Y, Liu LX, Chen YM, Xi F. Supramolecular hydrogels as a universal scaffold for stepwise delivering Dox and Dox/cisplatin loaded block copolymer micelles. Int J Pharm. 2012;437:11–9. https://doi.org/10.1016/j.ijpharm.2012.08.007
Master AM, Rodriguez ME, Kenney ME, Oleinick NL, Gupta AS. Delivery of the photosensitizer Pc 4 in PEG-PCL micelles for in vitro PDT studies. J Pharm Sci. 2010;99:2386–98. https://doi.org/10.1002/jps.22007
Li J, Li X, Ni X, Wang X, Li H, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO-PHB-PEO triblock copolymers and alpha-cyclodextrin for controlled drug delivery. Biomaterials . 2006;27:4132–40. https://doi.org/10.1016/j.biomaterials.2006.03.025
Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature . 1997;388:860–2. https://doi.org/10.1038/42218
Guo MY, Jiang M, Pispas S, Yu W, Zhou CX. Supramolecular hydrogels made of end-functionalized low-molecular-weight PEG and alpha-cyclodextrin and their hybridization with SiO2 nanoparticles through host-guest interaction. Macromolecules. 2008;41:9744–9. https://doi.org/10.1021/ma801975s
Feng RL, Zhu WX, Song ZM, Zhao LY, Zhai GX. Novel star-type methoxy-poly(ethylene glycol) (PEG)-poly(epsilon-caprolactone) (PCL) copolymeric nanoparticles for controlled release of curcumin. J Nanopart Res. 2013;15:1748–59. https://doi.org/10.1007/S11051-013-1748-5
Zhao SP, Lee JH, Xu WL. Supramolecular hydrogels formed from biodegradable ternary COS-g-PCL-b-MPEG copolymer with alpha-cyclodextrin and their drug release. Carbohydr Res. 2009;344:2201–8. https://doi.org/10.1016/j.carres.2009.08.017
Khodaverdi E, Aboumaashzadeh M, Tekie FSM, Hadizadeh F, Tabassi SAS, Mohajeri SA, et al. Sustained drug release using supramolecular hydrogels composed of cyclodextrin inclusion complexes with PCL/PEG multiple block copolymers. Iran Polym J. 2014;23:707–16. https://doi.org/10.1007/s13726-014-0265-4
Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6th edn. 2215 Constitution Avenue NW. Washington, DC: Pharmaceutical Press, American Pharmacists Association; 2009.
Miller T, van Colen G, Sander B, Golas MM, Uezguen S, Weigandt M, et al. Drug loading of polymeric micelles. Pharm Res. 2013;30:584–95. https://doi.org/10.1007/s11095-012-0903-5
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–76. https://doi.org/10.1016/S0731-7085(96)02024-9
Pradhan M, Singh D, Singh MR. Novel colloidal carriers for psoriasis: current issues, mechanistic insight and novel delivery approaches. J Control Release. 2013;170:380–95. https://doi.org/10.1016/j.jconrel.2013.05.020
Firooz A, Nafisi S, Maibach HI. Novel drug delivery strategies for improving econazole antifungal action. Int J Pharm. 2015;495:599–607. https://doi.org/10.1016/j.ijpharm.2015.09.015
Klaewklod A, Tantishaiyakul V, Hirun N, Sangfai T, Li L. Characterization of supramolecular gels based on beta-cyclodextrin and polyethyleneglycol and their potential use for topical drug delivery. Mater Sci Eng C Mater Biol Appl. 2015;50:242–50. https://doi.org/10.1016/j.msec.2015.02.018
Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2008;70:633–40. https://doi.org/10.1016/j.ejpb.2008.05.008
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province [Grant number ZR2016BL15]; Science and Technology Project of University of Jinan [Grant number XKY1732]; and Shandong Talents Team Cultivation Plan of University Preponderant Discipline [Grant number 10027].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, F., Song, Z., Wen, Y. et al. Transdermal delivery of curcumin-loaded supramolecular hydrogels for dermatitis treatment. J Mater Sci: Mater Med 30, 11 (2019). https://doi.org/10.1007/s10856-018-6215-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6215-5