Abstract
The structure of ice-templated collagen scaffolds is sensitive to many factors. By adding 0.5 wt% of sodium chloride or sucrose to collagen slurries, scaffold structure could be tuned through changes in ice growth kinetics and interactions of the solute and collagen. With ionic solutes (sodium chloride) the entanglements of the collagen molecule decreased, leading to fibrous scaffolds with increased pore size and decreased attachment of chondrocytes. With non-ionic solutes (sucrose) ice growth was slowed, leading to significantly reduced pore size and up-regulated cell attachment. This highlights the large changes in structure and biological function stimulated by solutes in ice-templating systems.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Within tissue engineering, collagen scaffold architecture is key for determining the biological activity of a scaffold [1, 2]. The architecture is defined by the scaffold isotropy, whether it possesses pores with inherent directionality or is purely isotropic, and by the pore size. Both architectural features can be modified using ice-templating techniques, which are controlled by the crystallization of ice within an aqueous solution [3]. Scaffold anisotropy is determined at nucleation, while the final pore size is linked to crystal growth and annealing [4, 5].
Ice nucleation and growth are sensitive to many factors, and literature has focused on altering set freezing protocols and mold design [1, 5]. In addition to these factors, ice crystallization is also sensitive to the amount and type of solutes present [3]. Solutes, such as sucrose and salts, slow ice crystal growth as their concentration in solution increases [6, 7]. Macromolecular solutes also decrease the ice growth rate as the concentration and the polymer molecular weight increase, an effect largely independent of viscosity [8]. Collagen slurry incorporates both types of solutes, consisting of insoluble collagen fibrils suspended within an aqueous solution. In practice, increasing collagen concentration within slurries leads to decreased pore size [4]. However, the composition of the aqueous solution and how it affects collagen slurry behavior, scaffold structure, and bioactivity has not been studied. It was hypothesized that the structure of collagen scaffolds could be modified with the addition of solutes. Two solutes were chosen: sodium chloride (NaCl), which is known to impact collagen structure and ice formation, and sucrose, which is a well studied non-ionic solute used as a model system to understand ice growth kinetics [6, 9]. Four suspensions of 1 wt% collagen were compared: no additives, 0.5 wt% sodium chloride (NaCl), 0.5 wt% sucrose, and 5 wt% sucrose, and the biological activity was tested in vitro using chondrocytes.
2 Experimental
2.1 Collagen slurry
Collagen slurry (1 wt%) was prepared by hydrating acid-insoluble bovine Achilles tendon, type I collagen (Sigma). in 0.05 M acetic acid containing: no additives, 0.5 wt% sucrose, 5 wt% sucrose and 0.5 wt% sodium chloride (NaCl). All slurries, adjusted to pH 2, were homogenized and frozen in stainless steel molds (filling height 5 mm). The slurry was cooled, at 0.9 °C/min, to −30 °C, held for 90 min, then lyophilized (Virtis, SP Industries). Scaffolds were washed for 1 h in deionized water and cross-linked via N-(3-dimethylamino propyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS) for 2 h in 70 % ethanol.
2.2 Scaffold characterization
Microscopy images were taken using a JEOL 820 (10 kV). X-ray tomography (Skyscan 1172) scans were used for pore sizing [4]. The pixel size for scans was 2.1 µm, and reconstructions were performed with NRecon (Skyscan). Average pore size was determined by line-intercept method [4].
2.3 Slurry rheology
Rheological measurement was done using an ARES rheometer at 4 °C using strain sweeps and frequency sweeps.
2.4 Cell isolation and seeding
Human chondrocytes were isolated from tissue removed during joint replacement. Ethical approval was obtained from Cambridgeshire 2 Research Ethics Committee. Briefly, minced cartilage was digested for 2 h in 0.2 % collagenase (Roche), at 37 °C, in complete media (10 % fetal bovine serum, 2 mM glutamine, 1 µg/ml penicillin/streptomycin, 0.001 µg/ml Amphotericin, 0.01 µg/ml gentamycin). Cells were washed in media, plated in monolayer culture, and used below passage 3.
Scaffolds with no additives, with 0.5 wt% sucrose and 0.5 wt% NaCl were tested. All scaffolds (5 × 5 × 2 mm) were sterilized for 1 h in 70 % ethanol and blotted on filter paper (Whatman) before seeding with 1 × 105 chondrocytes in 10 µl of complete media. Cells were incubated (5 % CO2, 37 °C) for 2 h for cell attachment before flooding the wells.
2.5 Biological characterization
Cell number was evaluated via the Hoechst assay, after 1, 7, and 14 days [10]. Constructs were digested at 60 °C in papain buffer [11]. Briefly, 10 µl of papain digest was added to Hoechst dye (Bisbenzimide H) for a final dye concentration of 0.1 µg/ml and fluorescence was measured (360 nm Ex, 460 nm Em). Metabolic activity was measured using an alamarBlue® assay (Invitrogen, UK) over 14 days. Samples were incubated for 4 h (37 °C) in 10 % alamarBlue® and fluorescence was read.
Protein expression at 14 days was analyzed using SDS-PAGE and Western blotting, as in Hernandez et al. [12]. Scaffolds were incubated in cell lysis buffer (Invitrogen) and mixed with sodium dodecyl-sulphate (SDS) sample buffer. Standards were prepared from human cartilage, snap frozen in liquid nitrogen, milled using a dismembrator (B Braun Biotech International GmbH), and suspended in cell lysis buffer (100 mg/ml). Scaffolds were probed for fibronectin (SC-9068, Santa Cruz Biotech), then stripped and reprobed for GAPDH as a control. Membranes were developed using ECL prime reagents (Amersham) and signal strength assessed using a densitometer.
3 Results
The collagen reacted strongly to the addition of an ionic solute, and after 0.5 wt% NaCl, the insoluble flakes came out of suspension. Thus 0.5 wt% was the limit of NaCl addition. Electrostatic interactions play a large role in the fibrillogenesis of collagen, with ionic additions retarding the rate of fibrillogenesis and altering the charge of collagen molecules within solutions [9, 13]. While the insoluble collagen used in the current study should not undergo fibrillogenesis, the charge on the molecules would still be sensitive to the addition of NaCl. In the case of sucrose addition, while the collagen remained in suspension after 5 wt% sucrose, excess sucrose crystallized onto the scaffold pore walls, Fig. 1. After washing the scaffold to remove the sucrose, the collagen structure remained brittle and inelastic, and thus, experiments were only carried out using 0.5 wt% sucrose.
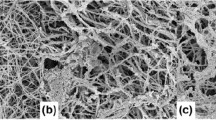
Collagen scaffold structure and the rheological properties of the slurry were affected by solute additions. Addition of 0.5 wt% NaCl changed the conformation of the collagen, altering a the viscosity of the slurry, and b the storage (G′) and elastic moduli (G″). Collagen scaffold structure with c no solute addition, d 0.5 wt% sucrose, and e 5 wt% sucrose and f 0.5 wt% NaCl; scale bar is 100 μm. The fibrillar structure of scaffolds with 0.5 wt% NaCl is clearly visible at higher magnification g ×400 and h ×3,300
Slurry additives affected the scaffold structure significantly, Fig. 1. With NaCl addition, the scaffold pore walls contained fine collagen fibrils, Fig. 1g, h, rather than the smooth walls in scaffolds with no addition or with 0.5 wt% sucrose. The range of mean pore size was also altered significantly. Addition of NaCl increased the range of pore sizes from 70–100 to 85–120 µm, while 0.5 wt% sucrose decreased the pore size distribution to 64–75 µm. The pore size within collagen scaffolds is related to the amount of ice growth and annealing which the structure undergoes around the equilibrium freezing temperature [5]. The addition of solutes such as sucrose or NaCl into an aqueous solution should reduce the rate of crystal growth and annealing [7]. However, the apparent size and shape of macromolecules also influences ice growth by mechanical hindrance at the crystal surface [8]. Reductions in the molecular weight of polymers, or the amount of entanglements among polymers, result in an increased ice growth rate [8].
In the current study, the final scaffold pore size was due to the combination of ice growth rate alteration and changes to the collagen molecule, highlighting the importance of solute interactions when designing collagen slurries for tissue engineering. With sucrose addition, which did not affect the behavior of the collagen, pore size was smaller due to decreased ice annealing. However, with ionic additions, the shape of the collagen molecule was changed, as seen by the viscosity of the slurry, which decreased from 36.3 to 3.2 Pa-s, suggesting the collagen had fewer entanglements, Fig. 1. The reduction of the molecular entanglements in the polymer offset the decrease in growth rate caused by salt addition and allowed the ice crystals to grow significantly larger. The addition of NaCl to the slurry also caused a significant decrease in the storage and loss moduli of the slurry.
3.1 Biological activity
The changes to scaffold structure with slurry additives were reflected in the biological activity of chondrocytes. The metabolic activity of the cells remained unchanged with the addition of 0.5 wt% NaCl or 0.5 wt% sucrose to the collagen slurries, but cell number was significantly affected, even 1 day after seeding, Fig. 2. As cell number appeared constant over the course of the study, solute addition appears to be altering the initial cell attachment. On all three scaffold types, chondrocytes retained the ability to adhere to the scaffolds, verified by the expression of fibronectin, an adhesive ECM protein, Fig. 2c.
Addition of solutes to the collagen slurry altered the biological activity. a Metabolic activity of chondrocytes, measured via an alamarBlue® assay, did not vary significantly. b Cell number was significantly impacted by solutes. c Protein expression of fibronectin, assessed using western blotting, with GAPDH expression below as a control; A cartilage tissue, B no additives, C 0.5 wt% NaCl, D 0.5 wt% sucrose. Significantly greater than all other samples (*P < 0.05). Significantly lower than all other samples (# P < 0.05)
Significant changes in cell attachment were apparent, but the relative contribution of scaffold architecture and chemical composition of the scaffold is not known. The cell culture medium itself contains high levels of both NaCl and sugars, making it unlikely that low levels of solute remaining in the scaffolds after washing were the cause of the biological changes. Instead, it has been shown in literature that cell attachment is highly dependent on the surface area for attachment, and thus the scaffold pore size [1]. Addition of 0.5 wt% sucrose to the scaffold decreased pore size and thus increased cell number significantly, while 0.5 wt% NaCl addition decreased cell number. The fibrillar structure of scaffolds with NaCl might also affect cell metabolism, as cells are known to respond to structural changes in their micro-environments, which could account for the lack of significant difference in metabolic activity on scaffolds with salt [14].
Tailoring cell response by altering the properties of tissue engineering scaffolds is a major focus of regenerative medicine. Of the many properties which can be varied within ice-templated scaffolds, architectural cues have been shown to have a powerful effect on biological activity [2, 14]. The architecture of ice-templated scaffolds is dependent on the nucleation and growth of ice within the structure, which is, in turn, controlled by factors such as freezing protocol, mold design and slurry composition [3]. Research on collagen scaffolds has focused on altering the polymeric component of the slurry [15]. However, the composition of the aqueous solution also plays an important role in the slurry, as shown in the current study. The addition of NaCl and sucrose were found to affect the scaffold architecture, and therefore, the biological activity of chondrocytes, especially initial cell attachment. This study offers a new route by which tissue engineering scaffolds and their biological properties can be tailored.
4 Conclusions
The structure of ice-templated collagen scaffolds is controlled by the growth of ice during the solidification process. Ice crystallization is sensitive to many factors, including the composition of collagen slurry. In the current study, 0.5 wt% of sodium chloride or sucrose were added to collagen slurries. It was found that the final scaffold structure was influenced by both changes in ice growth kinetics and the interactions of the solute with the collagen. Sodium chloride, which reduced the viscosity of the slurry by decreasing the entanglements of the collagen molecule, led to scaffolds with significantly larger pore sizes and with fibrillar walls. Sucrose, which did not interact strongly with collagen, decreased the scaffold pore size by slowing ice crystal growth. Changing scaffold structure with additives also impacted the biological activity of the scaffolds. Addition of sodium chloride decreased the cell attachment of chondrocytes on the scaffolds significantly, while sucrose addition had the opposite effect. This study highlights the large changes in structure and biological function which can be stimulated by altering the aqueous component of collagen slurries.
References
O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-gag scaffolds. Biomaterials. 2005;26(4):433–41.
Pawelec KM, Wardale J, Best SM, Cameron RE. The effects of scaffold architecture and fibrin gel addition on tendon cell phenotype. J Mater Sci. 2015;26:1–10.
Pawelec KM, Husmann A, Best SM, Cameron RE. Ice-templated structures for biomedicaltissue repair: from physics to final scaffolds. Appl Phys Rev. 2014;1:021301.
Pawelec KM, Husmann A, Best SM, Cameron RE. Understanding anisotropy and architecture in ice-templated biopolymer scaffolds. Mater Sci Eng C. 2014;37:141–7.
Pawelec KM, Husmann A, Best SM, Cameron RE. A design protocol for tailoring ice-templated scaffold structure. J R Soc Interface. 2014;11:20130958.
Hindmarsh JP, Russell AB, Chen XD. Measuring dendritic growth in undercooled sucrosesolution droplets. J Cryst Growth. 2005;285(1–2):236–48.
Pronk P, Hansen TM, Ferreira CAI, Witkamp GJ. Time-dependent behavior of different iceslurries during storage. Int J Refrig. 2005;28(1):27–36.
Blond G. Velocity of linear crystallization of ice in macromolecular systems. Cryobiology. 1988;25(1):61–6.
Wood GC, Keech MK. Formation fibrils from collagen solutions 1. Effect of experimentalconditions—kinetic and electron-microscopy. Biochem J. 1960;75:588–98.
Kim Y-J, Sah RL, Doong J-YH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using hoechst 33258. Anal Biochem. 1988;174(1):168–76.
Farndale R, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphatedglycosaminoglycans by use of dimethylmehtylene blue. Biochem Biophys Acta. 1986;883(2):173–7.
Hernandez P, Whitty C, Wardale RJ, Henson FMD. New insights into the location and form of sclerostin. Biochem Biophys Res Commun. 2014;446:1108–13.
Li Y, Asadi A, Monroe M, Douglas EP. pH effects on collagen fibrillogenesis in vitro: electrostatic interactions and phosphate binding. Mater Sci Eng C. 2009;29:1643–9.
Yin Z, Chen X, Chen JL, Shen WL, Nguyen TMH, Gao L, Ouyang HW. The regulationof tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163–75.
Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE. Collagen-hyaluronicacid scaffolds for adipose tissue engineering. Acta Biomater. 2010;6(10):3957–68.
Acknowledgments
The authors gratefully acknowledge the financial support of the Gates Cambridge Trust, the Newton Trust, NIHR, and ERC Advanced Grant 320598 3D-E. A.H. holds a Daphne Jackson Fellowship funded by the University of Cambridge. Also, the authors thank Dr. S. Butler for help with the rheological measurements.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pawelec, K.M., Husmann, A., Wardale, R.J. et al. Ionic solutes impact collagen scaffold bioactivity. J Mater Sci: Mater Med 26, 91 (2015). https://doi.org/10.1007/s10856-015-5457-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5457-8