Abstract
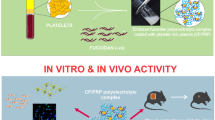
Recombinant human epidermal growth factor (rhEGF) is known to stimulate cell proliferation and accelerate wound healing. Direct delivery of rhEGF at the wound site in a sustained and controllable way without loss of bioactivity would enhance its biological effects. The aim of this study was to prepare a novel local delivery system for the sustained and controllable release of rhEGF, a fibrin gel loaded with chitosan nanoparticles. First, rhEGF-loaded chitosan nanoparticles were prepared and characterized, and these showed an ability to protect rhEGF from proteolysis. The prepared nanoparticles were then incorporated into a fibrin gel matrix during polymerization. In vitro release studies showed that the fibrin gel loaded with rhEGF/chitosan nanoparticles could achieve a more sustained release of rhEGF than either chitosan nanoparticles or an unloaded fibrin gel. Additionally, the release rate could be controlled by altering the contents of fibrinogen and thrombin in this composite delivery system. The bioactivity of the released rhEGF was determined by assessing its ability to stimulate the proliferation of BALB/c 3T3 cells, and the results showed that rhEGF bioactivity was not affected during the preparation process and could be maintained for at least 7 days. This novel delivery system may have great potential applications in the local administration of rhEGF.







Similar content being viewed by others
References
DiBiase MD, Rhodes CT. The design of analytical methods for use in topical epidermal growth factor product development. J Pharm Pharmacol. 1991;43(8):553–8.
Kitajima T, Sakuragi M, Hasuda H, Ozu T, Ito Y. A chimeric epidermal growth factor with fibrin affinity promotes repair of injured keratinocyte sheets. Acta Biomater. 2009;5(7):2623–32.
Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, et al. Wound healing: the role of growth factors. Drugs Today. 2003;39(10):787–800.
Mössner J, Caca K. Developments in the inhibition of gastric acid secretion. Eur J Clin Invest. 2005;35(8):469–75.
Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–52.
Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HP-[beta]-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. 2002;233(1–2):159–67.
Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009;5(7):2570–8.
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28.
Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med. 2009;20(5):1057–79.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75(1):1–18.
Zhang XG, Teng DY, Wu ZM, Wang X, Wang Z, Yu DM, et al. PEG-grafted chitosan nanoparticles as an injectable carrier for sustained protein release. J Mater Sci Mater Med. 2008;19(12):3525–33.
Hasanovic A, Zehl M, Reznicek G, Valenta C. Chitosan-tripolyphosphate nanoparticles as a possible skin drug delivery system for aciclovir with enhanced stability. J Pharm Pharmacol. 2009;61(12):1609–16.
Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell in growth matrix. J Control Release. 2000;69(1):149–58.
Kaufman MR, Westreich R, Ammar SM, Amirali A, Iskander A, Lawson W. Autologous cartilage grafts enhanced by a novel transplant medium using fibrin sealant and fibroblast growth factor. Arch Facial Plast Surg. 2004;6(2):94–100.
Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF–fibrin matrices for endothelialization. J Control Release. 2001;72(1–3):101–13.
Giannoni P, Hunziker EB. Release kinetics of transforming growth factor-beta1 from fibrin clots. Biotechnol Bioeng. 2003;83(1):121–3.
Gwak SJ, Kim SS, Sung K, Han J, Choi CY, Kim BS. Synergistic effect of keratinocyte transplantation and epidermal growth factor delivery on epidermal regeneration. Cell Transplant. 2005;14(10):809–17.
Briganti E, Spiller D, Mirtelli C, Kull S, Counoupas C, Losi P, et al. A composite fibrin-based scaffold for controlled delivery of bioactive pro-angiogenetic growth factors. J Control Release. 2010;142(1):14–21.
Park JS, Yoon JI, Li H, Moon DC, Han K. Buccal mucosal ulcer healing effect of rhEGF/Eudispert hv hydrogel. Arch Pharm Res. 2003;26(8):659–65.
Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32(3):319–27.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835.
Hardwicke J, Ferguson EL, Moseley R, Stephens P, Thomas DW, Duncan R. Dextrin-rhEGF conjugates as bioresponsive nanomedicines for wound repair. J Control Release. 2008;130(3):275–83.
Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing–past, present and future perspectives. Surgeon. 2008;6(3):172–7.
Yang CH, Huang YB, Wu PC, Tsai YH. The evaluation of stability of recombinant human epidermal growth factor in burn-injured pigs. Process Biochem. 2005;40(5):1661–5.
Buckley A, Davidson JM, Kamerath CD, Woodward SC. Epidermal growth factor increases granulation tissue formation dose dependently. J Surg Res. 1987;43(4):322–8.
Hori K, Sotozono C, Hamuro J, Yamasaki K, Kimura Y, Ozeki M, et al. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release. 2007;118(2):169–76.
Yang CH. Evaluation of the release rate of bioactive recombinant human epidermal growth factor from crosslinking collagen sponges. J Mater Sci Mater Med. 2008;19(3):1433–40.
Zhang S, Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26(7):1561–80.
Schultz CL, Morck DW. Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clin Exp Optom. 2010;93(2):61–5.
Cetin M, Aktas Y, Vural I, Capan Y, Dogan LA, Duman M, et al. Preparation and in vitro evaluation of bFGF-loaded chitosan nanoparticles. Drug Deliv. 2007;14(8):525–9.
Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82.
Masuoka K, Ishihara M, Asazuma T, Hattori H, Matsui T, Takase B, et al. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials. 2005;26(16):3277–84.
Zhou S, Deng X, Li X. Investigation on a novel core-coated microspheres protein delivery system. J Control Release. 2001;75(1–2):27–36.
Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J Control Release. 2010;148(1):49–55.
Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105(3):249–59.
Lee SB, Geroski DH, Prausnitz MR, Edelhauser HF. Drug delivery through the sclera: effects of thickness, hydration, and sustained release systems. Exp Eye Res. 2004;78(3):599–607.
Sekiyama E, Nakamura T, Kurihara E, Cooper LJ, Fullwood NJ, Takaoka M, et al. Novel sutureless transplantation of bioadhesive-coated, freeze-dried amniotic membrane for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2007;48(4):1528–34.
Acknowledgments
This work was supported by grants from the Chongqing Science and Technology Committee (CSTC, 2008AC0086) and the First Affiliated Hospital of Chongqing Medical University (YXJJ2009-09).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, W., Zhao, M., Zhao, Y. et al. A fibrin gel loaded with chitosan nanoparticles for local delivery of rhEGF: preparation and in vitro release studies. J Mater Sci: Mater Med 22, 1221–1230 (2011). https://doi.org/10.1007/s10856-011-4304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4304-9