Abstract
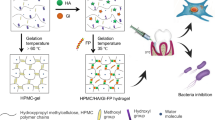
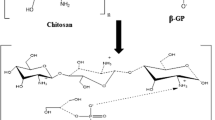
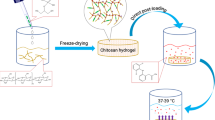
A novel injectable thermosensitive hydrogel (CS–HTCC/α β-GP) was successfully designed and prepared using chitosan (CS), quaternized chitosan (HTCC) and α,β-glycerophosphate (α,β-GP) without any additional chemical stimulus. The gelation point of CS–HTCC/α β-GP can be set at a temperature close to normal body temperature or other temperature above 25°C. The transition process can be controlled by adjusting the weight ratio of CS to HTCC, or different final concentration of α,β-GP. The optimum formulation is (CS + HTCC) (2% w/v), CS/HTCC (5/1 w/w) and α,β-GP 8.33% or 9.09% (w/v), where the sol–gel transition time was 3 min at 37°C. The drug released over 3 h from the CS–HTCC/α,β-GP thermosensitive hydrogel in artificial saliva pH 6.8. In addition, CS–HTCC/α,β-GP thermosensitive hydrogel exhibited stronger antibacterial activity towards two periodontal pathogens (Porphyromonas gingivalis, P.g and Prevotella intermedia, P.i). CS–HTCC/α, β-GP thermosensitive hydrogel was a considerable candidate as a local drug delivery system for periodontal treatment.




Similar content being viewed by others
References
Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm. 2008;365:89–99. doi:10.1016/j.ijpharm.2008.08.027.
Zhou HY, Chen XG, Kong M, Liu CS, Cha DS, Kennedy JF. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr Polym. 2008;73:265–73. doi:10.1016/j.carbpol.2007.11.026.
Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and haemostatic potential. J Biomed Mater Res. 1997;34:21–8. doi:10.1002/(SICI)1097-4636(199701)34:1<21::AID-JBM4>3.0.CO;2-P.
Molinaro G, Leroux JC, Damas J, Adam A. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23:2717–22. doi:10.1016/S0142-9612(02)00004-2.
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi:10.1016/j.biotechadv.2007.07.009.
Schmitz T, Grabovac V, Palmberger TF, Hoffer MH, Bernkop-Schnurch A. Synthesis and characterisation of a chitosan-n-acetyl cysteine conjugate. Int J Pharm. 2008;347:79–85. doi:10.1016/j.ijpharm.2007.06.040.
Jumaa M, Furkert FH, Muller BW. A new lipid emulsion formulation with high antimicrobial efficacy using chitosan. Eur J Pharm Biopharm. 2002;53:115–23. doi:10.1016/S0939-6411(01)00191-6.
Kim KW, Thomas RL, Lee C, Park HJ. Antimicrobial activity of native chitosan, degraded chitosan, and O-carboxymethylated chitosan. J Food Prot. 2003;66:1495.
Lehr C, Bouwstra J, Schacht E, Junginger H. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–8. doi:10.1016/0378-5173(92)90353-4.
Ma ZW, Zhang YJ, Wang R, Wang QT, Dong GY, Wu ZF. An animal experiment for the regeneration of periodontal defect by application of the dual-release chitosan thermosensitive hydrogel system. Zhonghua kou Qiang Yi Xue Za Zhi. 2008;43:273–7.
Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103:609–24. doi:10.1016/j.jconrel.2004.12.019.
Lagarce F, Faisant N, Desfontis JC, Marescaux L, Gautier F, Richard J, et al. Baclofen-loaded microspheres in gel suspensions for intrathecal drug delivery: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2005;61:171–80. doi:10.1016/j.ejpb.2005.04.004.
Cho JH, Kim SH, Park KD, Jung MC, Yang WI, Han SW, et al. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials. 2004;25:5743–51. doi:10.1016/j.biomaterials.2004.01.051.
Chung H, Go D, Bae J, Jung I, Lee J, Park K. Synthesis and characterization of Pluronic® grafted chitosan copolymer as a novel injectable biomaterial. Curr Appl Phys. 2005;5:485–8. doi:10.1016/j.cap.2005.01.015.
Ganji F, Abdekhoda M. Synthesis and characterization of a new thermosensitive chitosan–PEG diblock copolymer. Carbohydr Polym. 2008;74:435–41. doi:10.1016/j.carbpol.2008.03.017.
Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–61. doi:10.1016/S0142-9612(00)00116-2.
Ruel-Gariepy E, Leclair G, Hildgen P, Gupta A, Leroux JC. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release. 2002;82:373. doi:10.1016/S0168-3659(02)00146-3.
Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–42. doi:10.1016/S0142-9612(99)00011-3.
Sandri G, Rossi S, Bonferoni MC, Ferrari F, Zambito Y, Di Colo G, et al. Buccal penetration enhancement properties of N-trimethyl chitosan: influence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm. 2005;297:146–55.
van Winkelhoff AJ, Herrera Gonzales D, Winkel EG, Dellemijn-Kippuw N, Vandenbroucke-Grauls CM, Sanz M. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol. 2000;27:79–86. doi:10.1034/j.1600-051x.2000.027002079.x.
Harrison JW, Svec TA. The beginning of the end of the antibiotic era? Part I. The problem: abuse of the “miracle drugs”. Quintessence Int. 1998;29:151–62.
Harrison JW, Svec TA. The beginning of the end of the antibiotic era ? part ii. Proposed solutions to antibiotic abuse. Quintessence Int. 1998;29:223–9.
Slots J, Pallasch TJ. Dentists’ role in halting antimicrobial resistance. J Dent Res. 1996;75:1338–41. doi:10.1177/00220345960750060201.
Loesche WJ. Antimicrobials in dentistry: with knowledge comes responsibility. J Dent Res. 1996;75:1432–3. doi:10.1177/00220345960750070101.
Sanai Y, Persson GR, Starr JR, Luis HS, Bernardo M, Leitao J, et al. Presence and antibiotic resistance of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens in children. J Clin Periodontol. 2002;29:929–34. doi:10.1034/j.1600-051X.2002.291008.x.
Walker CB. The acquisition of antibiotic resistance in the periodontal microflora. Periodontol. 1996;10:79–88. doi:10.1111/j.1600-0757.1996.tb00069.x.
Chen XG, Zheng L, Wang Z, Lee CY, Park HJ. Molecular affinity and permeability of different molecular weight chitosan membranes. J Agric Food Chem. 2002;50:5915–8. doi:10.1021/jf020151g.
Xu H, Kaar JL, Russell AJ, Wagner WR. Characterizing the modification of surface proteins with poly(ethylene glycol) to interrupt platelet adhesion. Biomaterials. 2006;27:3125–35. doi:10.1016/j.biomaterials.2006.01.012.
Chung YM, Simmons KL, Gutowska A, Jeong B. Sol–gel transition temperature of PLGA-g-PEG aqueous solutions. Biomacromolecules. 2002;3:511–6. doi:10.1021/bm0156431.
ISO TR 10271. Bern: printed in Switzerland, (1993).
National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria: approved standard M11–A5. 5th ed. Wayne, PA, USA: National Committee for Clinical Laboratory Standards; 2001.
National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7–A5. 5th ed. Wayne, PA, USA: National Committee for Clinical Laboratory Standards; 2000.
Jia Z, shen D, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001;333:1–6. doi:10.1016/S0008-6215(01)00112-4.
Qin CQ, Xiao L, DU YM, Shi XW, Chen JW. A new cross-linked quaternized-chitosan resin as the support of borohydride reducing agent. Reactive Funct Polymers. 2002;50:165–71. doi:10.1016/S1381-5148(01)00111-0.
Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–32. doi:10.1016/j.biomaterials.2006.12.024.
Acknowledgments
We would like to thank the financial support of International S&T Cooperation Program of China (2008DFA31640); Ministry of Education of the People’s Republic of China (20070423013); the Natural Science Foundation of Shandong Province (No. Y2006C110) and the Youth Foundation of Health Department of Shandong Province (No. 2007QZ021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, Q.X., Chen, X.G., Zhao, Q.S. et al. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J Mater Sci: Mater Med 20, 1603–1610 (2009). https://doi.org/10.1007/s10856-009-3729-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-009-3729-x