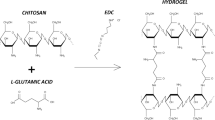
Abstract
In this study, diclofenac sodium (DS) is post-loaded directly into thermo-sensitive hydrogels fabricated from chitosan and β-glycerophosphate (GP) for temperature-controlled-release application at the inflammatory sites. Experiments analyzing the effect of GP concentration and simulated physiological temperature show that the hydrogels have a compatible heat-sensitive response through sol-gel and swelling behaviors. The hydrogels are soluble at 25 °C and rapidly gel above 37 °C with a decrease in the gelation time from 4 to 1 min when the temperature rises to 41 °C. Specific surface area, pore size, and pore volume of developed chitosan hydrogels are respectively 16.91–34.62 m2/g, 4.16–4.61 nm, and 0.08–0.28 cm3/g. Moreover, all hydrogels exhibit adaptive swelling to temperature changes as evidenced by the increase in equilibrium swelling ratio from 18.4–66.0% (25 °C) to 34.8–88.5% (37 °C) and 56.0–108.4% (39 °C). In terms of DS delivery, the highest loading capacity of the developed hydrogel is achieved at 8.81 mg/g with the GP content of 25 w/v%, the initial drug concentration of 2000 µg/ml, and the drug dissolution solvent being water, indicating the 23.8 times higher DS loading capacity compared with co-crosslinked chitosan/GP/genipin hydrogels in the previous work. About 87 and 95% of the loaded DS amount is released from the prepared chitosan hydrogels after 5 h at 37 and 39 °C correspondingly. The findings demonstrate that the ability to carry and release the anti-inflammatory DS of the thermo-sensitive chitosan hydrogels is significantly influenced by many subjective (composition) and objective (media temperature, initial drug concentration, and drug dissolution solvent) factors which can be adjusted to monitor the DS delivery at the right time and the right dose as required.
Graphical Abstract

Highlights
-
Diclofenac sodium is directly post-loaded into thermo-sensitive chitosan hydrogel.
-
The hydrogel is soluble at 25 °C and rapidly becomes gel at 39 °C in 4 min.
-
The highest swelling ratio of the hydrogel is 108.4% at 39 °C.
-
The maximum drug loading of the hydrogel is 8.81 mg/g.
-
87% and 95% of drug-loaded is released after 5 h at 37 °C and 39 °C, respectively.








Similar content being viewed by others
References
Supper S, Anton N, Seidel N, Riemenschnitter M, Curdy C, Vandamme T (2014) “Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications”. Expert Opin Drug Deliv 11:249–267. https://doi.org/10.1517/17425247.2014.867326
Jiang T, James R, Kumbar SG, and Laurencin CT (2014) “Chapter 5 - Chitosan as a biomaterial: Structure, properties, and applications in tissue engineering and drug delivery,” in Natural and Synthetic Biomedical Polymers, S. G. Kumbar, C. T. Laurencin, and M. Deng Eds. Oxford: Elsevier, p. 98.
Kulkarni N, Jain P, Shindikar A, Suryawanshi P, Thorat N (2022) “Advances in the colon-targeted chitosan based multiunit drug delivery systems for the treatment of inflammatory bowel disease”. Carbohydr Polym 288:119351. https://doi.org/10.1016/j.carbpol.2022.119351
Mahdavinia GR, Karimi MH, Soltaniniya M, Massoumi B (2019) “In vitro evaluation of sustained ciprofloxacin release from κ-carrageenan-crosslinked chitosan/hydroxyapatite hydrogel nanocomposites”. Int J Biol Macromolecules 126:443–453. https://doi.org/10.1016/j.ijbiomac.2018.12.240
Mikušová V, Mikuš P (2021) Advances in chitosan-based nanoparticles for drug delivery. Int J Mol Sci 22:17. https://doi.org/10.3390/ijms22179652
Ways TMM, Lau WM, Khutoryanskiy VV (2018) “Chitosan and its derivatives for application in mucoadhesive drug delivery systems”. Polymers10:3. https://doi.org/10.3390/polym10030267.
Saeedi M et al. (2022) Customizing nano-chitosan for sustainable drug delivery”. J Controlled Release 350:175–192. https://doi.org/10.1016/j.jconrel.2022.07.038
Chatterjee S, Hui PC-l (2018) “Stimuli-responsive hydrogels: An interdisciplinary overview,” in Hydrogels: Smart Materials for Biomedical Applications: IntechOpen, 1–23.
Zhou HY, Jiang LJ, Cao PP, Li JB, Chen XG (2015) “Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications”. Carbohydr Polym 117:524–536. https://doi.org/10.1016/j.carbpol.2014.09.094
Garami A, Steiner AA, Romanovsky AA (2018) “Chapter 34 - Fever and hypothermia in systemic inflammation,” in Handbook of Clinical Neurology, 157, A. A. Romanovsky Ed.: Elsevier, 565–597.
Alsaif NA, Bhat MA, Al-Omar MA, Al-Tuwajiri HM, Naglah AM, Al-Dhfyan A (2020) “Synthesis of Novel Diclofenac Hydrazones: Molecular Docking, Anti-Inflammatory, Analgesic, and Ulcerogenic Activity”. J Chem 2020:4916726. https://doi.org/10.1155/2020/4916726
Banning M (2008) “Topical diclofenac: Clinical effectiveness and current uses in osteoarthritis of the knee and soft tissue injuries”. Expert Opin Pharmacother 9:2921–2929. https://doi.org/10.1517/14656566.9.16.2921
Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O (2017) “Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries”. J Mater Chem B 5:9452–9476. https://doi.org/10.1039/C7TB01689A
Hu L et al. (2022) Construction of chitosan-based asymmetric antioxidant and anti-inflammatory repair film for acceleration of wound healing”. Int J Biol Macromolecules 215:377–386. https://doi.org/10.1016/j.ijbiomac.2022.06.103
Amani H et al. (2019) Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling”. Sci Rep 9:6044. https://doi.org/10.1038/s41598-019-42633-9
Tan LS et al. (2021) “Fabrication of radiation cross-linked diclofenac sodium loaded carboxymethyl sago pulp/chitosan hydrogel for enteric and sustained drug delivery”. Carbohydr Polym Technol Appl 2:100084. https://doi.org/10.1016/j.carpta.2021.100084
Gull N et al. (2020) Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium”. Int J Biol Macromolecules 162:175–187. https://doi.org/10.1016/j.ijbiomac.2020.06.133
Qi X et al. (2016) “Intra-articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium-Loaded Alginate Microspheres”. J Pharm Sci 105:122–130. https://doi.org/10.1016/j.xphs.2015.11.019
Tang Y, Zhao Y, Li Y, Du Y (2010) “A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing nanoparticles for drug delivery”. Polym Bull 64:791–804. https://doi.org/10.1007/s00289-009-0214-0
Maiz-Fernández S et al. (2020) β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery”. Carbohydr Polym 248:116811. https://doi.org/10.1016/j.carbpol.2020.116811
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) “Preparation and antibacterial activity of chitosan nanoparticles”. Carbohydr Res 339:2693–2700. https://doi.org/10.1016/j.carres.2004.09.007
Mazaheri M, Akhavan O, Simchi A (2014) “Flexible bactericidal graphene oxide–chitosan layers for stem cell proliferation”. Appl Surf Sci 301:456–462. https://doi.org/10.1016/j.apsusc.2014.02.099
Ning F, Zhang J, Kang M, Ma C, Li H, Qiu Z (2021) “Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent”. J Appl Polym Sci 138:49880. https://doi.org/10.1002/app.49880
Bo J, Luo X, Huang H, Li L, Lai W, Yu X (2018) “Morphology-controlled fabrication of polypyrrole hydrogel for solid-state supercapacitor”. J Power Sources 407:105–111. https://doi.org/10.1016/j.jpowsour.2018.10.064
Wei B, Zou J, Pu Q, Shi K, Xu B, Ma Y (2022) “One-step preparation of hydrogel based on different molecular weights of chitosan with citric acid”. J Sci Food Agriculture 102:3826–3834. https://doi.org/10.1002/jsfa.11732
Boido M, Ghibaudi M, Gentile P, Favaro E, Fusaro R, Tonda-Turo C (2019) “Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment”. Sci Rep 9:6402. https://doi.org/10.1038/s41598-019-42848-w
L. Boles, C. Alexander, L. Pace, W. Haggard, J. Bumgardner, and J. Jennings (2018) “Development and evaluation of an injectable chitosan/β-glycerophosphate paste as a local antibiotic delivery system for trauma care”. J Functional Biomater 9, 4. https://doi.org/10.3390/jfb9040056
Ganji F, Abdekhodaie MJ, Ramazani ASA (2007) “Gelation time and degradation rate of chitosan-based injectable hydrogel”. J Sol-Gel Sci Technol 42:47–53. https://doi.org/10.1007/s10971-006-9007-1
Özkahraman B, Acar I, Güçlü G (2022) “Synthesis of N-vinylcaprolactam and methacrylic acid based hydrogels and investigation of drug release characteristics”, Polymer Bulletin. https://doi.org/10.1007/s00289-022-04301-3
Ostrowska-Czubenko J, Gierszewska M, Pieróg M (2015) “pH-responsive hydrogel membranes based on modified chitosan: water transport and kinetics of swelling”. J Polym Res 22:153. https://doi.org/10.1007/s10965-015-0786-3
Neufeld L, Bianco-Peled H (2017) “Pectin–chitosan physical hydrogels as potential drug delivery vehicles”. Int J Biol Macromolecules 101:852–861. https://doi.org/10.1016/j.ijbiomac.2017.03.167
Wang QZ et al. (2006) Protonation constants of chitosan with different molecular weight and degree of deacetylation”. Carbohydr Polym 65:194–201. https://doi.org/10.1016/j.carbpol.2006.01.001
Acknowledgements
NHND was funded by Vingroup JSC and supported by the Master, PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), Institute of Big Data, code VINIF.2021.TS.056. We also acknowledge the support of time and facilities from Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for this study.
Author contributions
NHND: Investigation, Formal analysis, Writing - original draft, Validation; THP: Methodology, Characterization; PKL: Validation, Writing - review & editing; ACH: Conceptualization, Visualization, Project administration, Data curation, Writing - review & editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Do, N.H.N., Pham, T.H., Le, P.K. et al. Thermo-responsive Chitosan/β-glycerophosphate hydrogels directly post-loading anti-inflammatory diclofenac sodium. J Sol-Gel Sci Technol 105, 451–460 (2023). https://doi.org/10.1007/s10971-022-06020-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-06020-7