Abstract
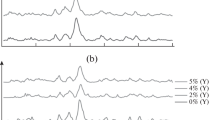
This study investigated the transformation of two sodium calcium borate glasses to hydroxyapatite (HA). The chemical reaction was between either 1CaO · 2Na2O · 6B2O3 or 2CaO · 2Na2O · 6B2O3 glass and a 0.25 M phosphate (K2HPO4) solution at 37, 75 and 200 °C. Glass samples in the form of irregular particles (125–180 μm) and microspheres (45–90 and 125–180 μm) were used in order to understand the reaction mechanism. The effect of glass composition (calcium content) on the weight loss rate and reaction temperature on crystal size, crystallinity and grain shape of the reaction products were studied. Carbonated HA was made by dissolving an appropriate amount of carbonate (K2CO3) in the 0.25 M phosphate solution. X-ray diffraction, Fourier transform infrared spectroscopy, and scanning electron microscopy were used to characterize the reaction products. The results show that sodium calcium borate glasses can be transformed to HA by reacting with a phosphate solution. It is essentially a process of dissolution of glass and precipitation of HA. The transformation begins from an amorphous state to calcium-deficient HA without changing the size and shape of the original glass sample. Glass with a lower calcium content (1CaO · 2Na2O · 6B2O3), or reacted at an elevated temperature (75 °C), has a higher reaction rate. The HA crystal size increases and grain shape changes from spheroidal to cylindrical as temperature increases from 37 to 200 °C. Increase in carbonate concentration can also decrease the crystal size and yield a more needle-like grain shape.










Similar content being viewed by others
References
L. L. HENCH and J. WILSON, An Introduction to Bioceramics, World Scientific, Singapore (1993)
N. PATEL, S. M. BEST, I. R. GIBSON, S. KE, K. A. HING and W. BONFIELD, Key Eng. Mater. 191–195 (2001) 7
L. L. HENCH, J. Am. Ceram. Soc. 74 (1991) 1487
I. REHMAN and W. BONFIELD, J. Mater. Sci. Mater. Med. 8 (1997) 1
D. G. A. NELSON and J. D. B. FEATHERSTONE, Calcif. Tissue Int. 34 (1982) 69
R. E. WUTHIER, G. S. RICE, J. E. B. WALLACE, R. L. WEAVER, R. Z. LEGEROS and E. D. EANES, Calcif. Tissue Int. 37 (1985) 401
K. A. HING, S. M. BEST and W. BONFIELD, J. Mater. Sci. Mater. Med. 10 (1999) 135
A. H. VERHOEF and H. W. D. HARTOG, J. Non-Cryst. Solids 182 (1995) 221
L. GUO, M. HUANG, Y. LENG, J. E. DAVES and X. ZHANG, Key Eng. Mater. 192–195 (2001) 187
C. DU, F. Z. CUI, K. D. GROOT and P. LAYROLLE, Key Eng. Mater. 218–220 (2002) 39
I. I. BARBA, A. J. SALINAS and M. V. REGI, J. Biomed. Mater. Res. 51 (2000) 191
M.T. PHAM, W. MATZ, H. REUTHER, E. RICHTER, G. STEINER and S. OSWALD, J. Biomed. Mater. Res. 59 (2002) 254
J. BARRALET, S. BEST and W. BONFIELD, J. Biomed. Mater. Res. 41 (1998) 79
I.R. GIBSON and W. BONFIELD, J. Biomed. Mater. Res. 59 (2002) 697
J.E. BARRALET, S. ALDRED, A. J. WRIGHT and A. G. A. COOMBES, J. Biomed. Mater. Res. 60 (2002) 360
S. D. CONZONE, R. F. BROWN, D. E. DAY and G. J. EHRHARDT, J. Biomed. Mater. Res. 60 (2002) 260
D. E. Clark, C. G. Pantano and L. L. Hench, Corrosion of Glass, Books for Industry and The Glass Industry (1979)
A. YASUKAWA, T. MATSUURA, M. NAKAJIMA, K. KADORI and T. ISHIKAWA, Mater. Res. Bull. 34 (1999) 589
Lee et al., US patent 6,117,456, September 12, 2000
P. W. BROWN and R. I. MARTIN, J. Phys. Chem. B. 103 (1999) 1671
B. J. MEENAN, A. BOYD, E. LOVE and M. AKAY, Key Eng. Mater. 192–195 (2001) 15
Z.H. CHENG, A. YASUKAWA, K. KANDORI and T. ISHIKAWA, Langmuir 14 (1998) 6681
D. J. GREENFIELD and E. D. EANES, Calcif. Tissue Res. 9 (1972) 152
Acknowledgment
The financial support from the UMR Graduate Center for Materials Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Day, D.E. Reaction of sodium calcium borate glasses to form hydroxyapatite. J Mater Sci: Mater Med 18, 1837–1847 (2007). https://doi.org/10.1007/s10856-007-3053-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3053-2