Abstract
This study examined the impact of hydrothermally synthesized TiO2:ZnO composite-based nanopowders on the efficiency of dye-sensitized solar cells (DSSCs). Pure TiO2, 3TiO2:1ZnO, 1TiO2:1ZnO, 1TiO2:3ZnO, and pure ZnO thin films were coated on the conductive glass surface by the screen printing method. XRD and SEM were used to examine the thin film’s surface photographs and crystal structure, respectively. XRD analyses confirmed the formation of targeted crystal phases in the structure. It was understood from SEM studies that the TiO2:ZnO composite structure was formed successfully. I-V characterization results showed that the cell combined with the composite 3TiO2:1ZnO electrode had the highest power conversion efficiency (9.9%). In addition, it was determined from EIS analysis that interfacial charge transfer occurred more efficiently in cells with high TiO2 ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Reducing the environmental damage of fossil fuels and overcoming global energy crises is only possible with innovative solutions. This highlights the significance of renewable energy sources, including solar energy [1]. Silicon-based solar cells are widely used in energy production, but their production is not cost-effective. Scientists have focused on new low-cost materials that can convert light energy directly into electrical energy. DSSCs are advantageous, thanks to their low cost, environmental friendliness, and simple production process compared to solar cells containing silicon [2, 3].
DSSCs consist of a metal oxide semiconductor layer coated on a conductive glass substrate, a counter electrode, and a redox electrolyte solution filling the gap between the two electrodes. The most important component of DSSCs is the photoanode sheet, which absorbs light and transfers the produced electrons to the circuit [4, 5]. Different nanostructures such as ZnO (zinc oxide), TiO2 (titanium dioxide), CuGaO2 (copper gallium oxide), Nb2O5 (niobium pentoxide), and SnO2 (tin oxide) have been frequently used in photoanodes recently [6, 7]. However, more effective electron transport and dye absorption are possible with the use of composite structured metal oxides such as MgO/TiO2 [8], TiO2/ZnO [9], Al2O3/TiO2 [10], and ZnO/SnO2 [11]. The main reason for this situation is the emergence of heterojunction structures in the metal oxide semiconductor thin film. These types of structures have the ability to minimize electron–hole pair recombination that occurs as a result of the excitation of photons. Nano-based composite materials are advantageous due to their features such as an economical production process and non-toxicity [12]. Recently, researchers have been trying to find the best combination to maximize the performance of nanocomposite-based DSSCs. Jung et al. reported that TiO2 electrode coated with MgO thin film showed 45% higher performance compared to bare TiO2 [13]. Lim et al. noted higher power conversion efficiency for Au–Ag-doped TiO2 electrode. This performance increase was explained to be the consequence of the synergistic impact between Gold(Au)-Silver(Ag), as well as the minimization of the charge recombination process [14]. In a similar study by Kanmani et al., higher open circuit voltage (Voc) was observed for TiO2/ZnO nanocomposites compared to pure TiO2 [15].
There are many studies on the use of different forms of composite-based metal oxides in DSSCs. Among these composites, TiO2/ZnO is important for DSSCs. TiO2 is singled out due to its economical synthesis process, good dye absorption capacity, long electron lifetime, stability, and optical properties [16]. In addition, the properties of ZnO such as non-toxicity, easy synthesis process, fast electron mobility, and nanocrystalline structure allowing good dye absorption encourage the use of this metal oxide in DSSCs. Due to these properties, ZnO and TiO2 are among the superior metal oxides in terms of photovoltaic power conversion efficiency. Besides, the high energy band gap of TiO2 and ZnO (TiO2 = 3.2 eV, ZnO = 3.37 eV) limits the photoconversion efficiency [17].
ZnO has higher electron mobility, but lower chemical stability, than TiO2 [18]. This situation causes lower cell efficiency of DSSCs using ZnO as metal oxide. Researchers are trying to overcome this impasse by using new coating methods, doping, and composite structures [19]. In particular, the high chemical stability of TiO2 and the faster electron mobility of ZnO have brought about the idea of combining the superior properties of both metal oxide semiconductors. Thanks to the composite structures in DSSCs, electron–hole transfer between the valence and conduction bands improves and the separation of charge carriers becomes easier [20]. Such behavior enhances the power conversion efficiency in DSCCs [9]. Yao et al. reported higher cell efficiency than pure TiO2 and ZnO using ZnO/TiO2 core–shell structure and nanorods in the photoanode. This is attributed to the fact that nanorods in ZnO provide a better diffusion path for electrons and reduce electron–hole recombination [21]. In a similar study, Park et al. observed higher cell performance in photoanodes using TiO2/ZnO composite structure, which was attributed to better dye absorption and stability [22].
In this study, hydrothermal method was used to produce TiO2/ZnO composite-based nanostructures. Pure and composite-based thin films were deposited on the conductive glass surface using the screen printing method. The structural properties of TiO2/ZnO composites prepared in volume ratios of 1:0, 1:1, 1:3, 3:1, and 0:1 were examined and their effects on DSSCs were investigated.
2 Experiments carried out for the study
2.1 Chemicals used for experiments
Most chemicals and reagents to be used in the study were purchased from Sigma-Aldrich and used in the experiments without purification. Zinc acetate dihydrate > % 99 pure:ACS reagent [Zn(CH3COO)2⋅2H2O, ≥ 99.0%] and hexamethylenetetramine [HMTA, C6H12N4] were used to produce ZnO nanorods. Titanium IV isopropoxide [TTIP, ≥ 97.0%] was used as the titanium source to synthesize TiO2 nanostructures. Ethyl cellulose and alpha-terpineol were used as binders for TiO2/ZnO paste production. Ultrapure water, methanol, and anhydrous ethanol were used as solvents. Fluorine-doped tin oxide [F:SnO2, 15 Ω/cm2] as a conductive glass substrate, platinum (Pt) for the photocathode, Ru-N719 as a sensitizing dye, AN-50 as a redox solution, and a sealing material were purchased from surlyn solaronix.
2.2 Production process of metal oxide powders
2.2.1 Production process of ZnO nanopowder
To prepare ZnO nanopowder, 0.1 M Zn(CH3COO)2⋅2H2O and 0.1 M HMTA were mixed until dissolved in two different glass bottles containing 40 ml of pure water. During the mixing process, HMTA was added dropwise to the solution prepared with zinc acetate and mixed at room conditions for 60 min. The resulting hazy solution was then placed in an autoclave lined with Teflon and heated to 180 °C for 4 h. Following the hydrothermal procedure, the resulting white particles were twice washed with ethanol and water in succession before being allowed to dry in an oven set at 80 °C. After completing the drying process in the oven, the white particles were pulverized with the help of an agate mortar and processed in a muffle furnace at 400 °C for 45 min.
2.2.2 Synthesis of TiO2 nanoparticles
To synthesize TiO2 nanostructures, 2.25 g of urea was mixed in 20 ml of pure water for 10 min. While the mixing process was continuing, 4 ml of TTIP was added dropwise to the urea solution and stirred at high speed for 20 min. To obtain a more homogeneous structure, this mixture was ultrasonically mixed at 100 Hz for 15 min. After the ultrasonic treatment, the mixture reached a milky consistency and was transferred to a Teflon-coated autoclave and left at 180 °C for 4 h. Following the autoclave's cooling to ambient temperature, the resulting white particles were twice washed with ethanol and water in succession before being dried in an oven set at 80 °C. After the white particles had finished drying in the oven, they were ground into a powder using an agate mortar and heated to 450 °C for 45 min in a muffle furnace.
2.2.3 Synthesis stages of TiO2/ZnO composite structures
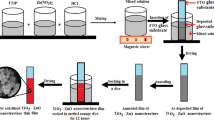
The precursor solutions prepared to synthesize TiO2 and ZnO were mixed in volume ratios of 1:1, 1:3, and 3:1 (v/v) and subjected to ultrasonic treatment for 20 min. The following steps are the same as the TiO2 and ZnO production process. Figure 1 shows the synthesis stages of nanoparticles and the production processes of DSSCs.
2.3 Preparation of DSSCs
In order to eliminate undesired residues, conductive glass substrates intended for use as working electrodes were cleaned in an ultrasonic bath using acetone, alcohol, and water, respectively. Pastes containing pure TiO2, pure ZnO, and different volumes of TiO2/ZnO were prepared using the same procedure. For this purpose, 0.25 g metal oxide nanopowder, 0.125 g ethyl cellulose, 2.5 ml α-terpineol, and 10 ml anhydrous ethanol were mixed in a closed glass bottle for 12 h at room temperature in a dark environment. To achieve the proper slurry consistency, part of the alcohol in the mixture was evaporated after the mixing procedure. The synthesized slurry-like pastes were deposited on the FTO glass surface by the screen printing method and dried at 80 °C for 1 h. The photoanodes were heat treated in a muffle furnace at 450 °C for 45 min to evaporate the binders in the paste structure. After being sintered and cooled in a desiccator to 60 °C, the samples were submerged in a 0.5 mM solution of dissolved Ruthenium N719 in methanol and left in a dark place for a day. Photoanodes sensitized with ruthenium dye were immersed in methanol solution several times to remove insoluble dyes from the surface and dried quickly. The electrode was covered with sealing material and combined with the Pt counter electrode. To join the electrodes, the sealing material was heat treated at 80 °C for 1 min. Photovoltaic measurements of DSSCs were carried out after adding the electrolyte solution through the opening on the Pt-coated counter electrode in the samples. On the FTO surface, the electrode's surface area is 0.36 cm2 and its layer thickness is 16 µm. Figure 2 shows parts of a representative DSSC.
2.4 Characterization
X-ray diffraction with a CuKα1 source (λ = 1.54 Å) was conducted using Rigaku MiniFlex-600 to investigate the minerological structures of metal oxide. EDS-coupled scanning electron microscopy (FE-SEM, Sigma 300) was used to capture the morphology of the nanostructures and key compositional features of the composite structures. Particle distribution analysis system (Betatek-zetatrac) was used to examine solid particle distribution. Absorption and reflectance measurements were carried out with a UV–vis spectrophotometer (UV-2400, Shimadzu) in the range of 200–1200 nm. Photovoltaic parameters of DSSCs were evaluated through a solar simulator (Fytronix solar simulator LSS 9000) under a 1.5 AM optical filter and 100 mW/cm2 artificial light intensity. Electrochemical Impedance Spectra (EIS) of the cells were characterized by an impedance analysis system (Fytronix EIS devices) in the frequency range of 0.1 Hz–100 kHz under 100 mW/cm2 light intensity.
3 Results and discussion
3.1 Investigation of structural features
The X-ray diffraction pattern of TiO2 powders synthesized using hydrothermal technique is shown in Fig. 3a. The crystal structure with the largest intensity peak is located in the (101) plane corresponding to 25.22°. In addition, the lower density (004), (200), (105), (211), (204), (116), (220), (215), and (224) planes corresponding to 2θ angles 37.74°, 48.02°, 53.82°, 55.10°, 62.56°, 68.82°, 70.18°, 75.18°, and 82.85°, respectively [23], confirm the anatase phase of TiO2 (JCPDS card no: 50-00-223). Figure 3b shows the diffraction pattern of ZnO metal oxide semiconductor produced by hydrothermal method. The main peaks of the ZnO metal oxide nanopowder in the (100), (002), (101), (102), (110), (103), (200), (112), (201), and (202) crystal planes were observed at 2θ degrees 31.79°, 34.54°, 36.29°, 47.23°, 56.58°, 62.85°, 66.35°, 67.92°, 69.10°, and 77.11°, respectively [24]. X-ray diffraction studies demonstrate that ZnO nanopowder has a hexagonal wurtzite phase (JCPDS card number: 36-1451). The sharp peak at 34.54° matches the ZnO (002) plane [25], which is attributed to the formation of one-dimensional nanorods. The absence of an additional peak outside the basic characteristics in the XRD models confirms that TiO2 and ZnO have high purity.
SEM images in Fig. 4 were utilized to examine TiO2/ZnO composite nanostructures synthesized at different volume ratios. The SEM image of pure TiO2 in Fig. 4a exhibits an arrangement of tightly interlocked spherical nanoparticles. The images of pure ZnO in Fig. 4b reflect the nanorod-like morphology in contact with each other. The images in Fig. 4c–e indicate that the composite structure was formed successfully in all samples. In the composite structure with a 3TiO2:1ZnO volume ratio in Fig. 4c, spherical TiO2 nanoparticles tightly surrounded the ZnO nanorods and such a heterostructure increases the power conversion efficiency and light harvesting in DSSCs [26]. Moreover, the elemental analysis of the composite nanostructures shown in Fig. 5 confirmed that the TiO2:ZnO ratios in different samples were compatible with each other. The presence of Si and In peaks in EDS graphs is attributed to the glass substrates of the samples.
3.2 Average particle distribution of metal oxide nanopowders
Figure 6 shows the particle size distribution of pure TiO2, pure ZnO, 3TiO2:1ZnO, 1TiO2:3ZnO, and 1TiO2:1ZnO metal oxide powders obtained using the Gaussian distribution function. With the help of Gaussian Distribution Function, it was observed that the average particle size of pure TiO2 was 65.66 nm, while that of pure ZnO metal oxide was 370.22 nm. The average particle distributions of composite-based 1TiO2:1ZnO (109.11 nm) and 1TiO2:3ZnO (211.19 nm) samples are between the particle distributions of pure TiO2 and pure ZnO. Remarkably, the average particle size of the composite 3TiO2:1ZnO sample is 47.53 nm, which is lower than that of pure TiO2 nanopowder. This may be due to the heterogeneous distribution of oxygen vacancies in the structure, lattice stresses created by different lattice structures, and interface interactions that cause distortions in the composite lattice [27]. In addition, the DSSC with the smallest average particle size in the photoanode exhibited the superior cell performance, which is due to the smaller average particle sizes in composite structures improve the electronic properties of the photoelectrode in DSSCs [28].
3.3 Optical properties of thin films
The optical properties of pure and composite-based photoanodes were analyzed to compare their absorption behavior and band gap energies. Figure 7a displays the absorbance spectra of TiO2, ZnO, and composite structured TiO2/ZnO samples in the wavelength range of 380–700 nm. It is evident that the composite 3TiO2:1ZnO thin film exhibits stronger absorbance than other samples in the wavelength range of 400–700 nm and that is a consequence of the emergence of new energy levels in the band gap of the composite-based thin film and the fact that it is more sensitive to light [29]. Furthermore, the relatively lower energy band gap (3.51 eV) of the photoanode with composite 3TiO2:1ZnO thin film compared to other samples was attributed to its greater absorption of light in the visible and near IR regions [30].
The Tauc formula in Eq. 1 was utilized to obtain the absorption coefficient of composite metal oxide semiconductor thin films using near optical transmittance. [31]. The energy band gap values of the thin films were obtained by interpolating a straight line on the energy axis (\(h\upsilon\)), as shown in Fig. 7b.
In this formula, A, Eg, h. and \(\upsilon\) represent an energy-independent constant, forbidden energy gap, plank constant, and frequency, respectively. The energy band gap values for ZnO and TiO2, 3TiO2:1ZnO, 1TiO2:1ZnO, and 1TiO2:3ZnO thin films were found to be 3.75, 3.60, 3.51, 3.63, and 3.66 eV, respectively. Calculated values are compatible with those reported in previous studies in the literature [32,33,34]. Compared to pure TiO2 and ZnO, the band gap value of the composite structured 3TiO2:1ZnO thin film is smaller. This is explained by the composite thin film's sophisticated crystal structure [21].
3.4 Jsc-V characteristics of DSSCs
Figure 8 shows the Jsc-V curves of ZnO thin film/dye, TiO2 thin film/dye, 3TiO2:1ZnO thin film/dye, 1TiO2:3ZnO thin film/dye, and 1TiO2:1ZnO thin film/dye-based cells. The photovoltaic characteristics of the manufactured DSSCs, including the open circuit voltage (Voc), short circuit current density (Jsc), fill factor (FF), and photoelectric conversion efficiency (Ƞ), are compiled in Table 1.
\(\eta\) and FF values of DSSCs were calculated with the help of Eq. 2, 3 given below [35].
In the equations above, Pmax and Pin represent maximum power and input power, respectively. DSSCs with different photoanodes exhibited different cell performances. The cell efficiencies of DSSCs with pure ZnO, pure TiO2, 3TiO2:1ZnO, 1TiO2:3ZnO, and 1TiO2:1ZnO thin films are 3.0%, 8.4%, 9.9%, 3.4%, and 6.8%, respectively. Among all DSSCs, the cell with composite 3TiO2:1ZnO thin film was calculated to have the highest power conversion efficiency (9.9%). This can be elucidated by the faster electron injection in the 3TiO2:1ZnO thin film compared to others and the surface morphology of tightly packed TiO2 nanospheres that densely surround the ZnO nanorods. The presence of tightly packed nanospheres enhances light absorption and facilitates more efficient electron–hole pair production [36]. Higher power conversion efficiencies are evident in cells with high TiO2 ratio. This difference may be due to factors such as particle size, stronger interaction of TiO2 with the dye, and stronger dye regeneration with TiO2 [37]. Pure ZnO or composite thin films with high ZnO content exhibit lower cell efficiency, likely due to the minimal overlap between the excited state of the dye and the ZnO conduction band [38]. DSCC combined with composite 3TiO2:1ZnO photoanode exhibited higher cell efficiency compared to pure TiO2, since the morphology in the composite structure reduces the energy band gap. This reduction in the energy band gap results in superior electrochemical behavior in the near-UV region. Additionally, this reduction in the energy band gap not only enables better dye absorption in this cell, but also provides superior cell performance [39].
3.5 Electrochemical impedance analyzes (EIS) of DSSCs
The interfacial charge transfer resistance properties of the prepared DSSCs were investigated using EIS. The solar simulator was set to a frequency range of 0.1–100,000 Hz, a light intensity of 100 mW/cm2, and the responses of the cells to a perturbation voltage of 10 mV were recorded. Nyquist curves and equivalent circuit model of DSSCs with pure ZnO, pure TiO2, 3TiO2:1ZnO, 1TiO2:1ZnO, and 1TiO2:3ZnO-based photoanodes are given in Fig. 9a. The parameters obtained from EIS analyses were edited with Zview software, and resistance parameters such as Rs (series resistance), Rct (interfacial charge transfer resistances), and \(\tau\)(electron lifetime) are shown in Table 2.
Nyquist curves are present in three different frequency regions. The semicircles on the far left of the graph are in the extremely high frequency region and related to the Rs of the photoanode. Rs is also known as the ohmic resistance resulting from the contact of the oxide layer and the electrolyte solution. The larger semicircles in the mid-range frequency region represent the Rct2 (charge transfer resistance) at the photoanode–redox electrolyte interface. The third semicircles in very low frequency region are associated with the Rct1 (Warburg diffusion resistance) [40]. Since the counter electrodes of all cells produced are Pt based, Rct1 values are very close to each other [35]. Accordingly Rct2 should be taken into account when examining the interface resistance parameters of cells. The lower Rs value of 3TiO2:1ZnO-based DSSC (15.19 Ω) compared to other cells is a consequence of the decrease in the interfacial charge transfer resistance at the between FTO/3TiO2:1ZnO/dye layers [41]. It is known that smaller semicircles in the mid-frequency region reflect lower Rct2 values. The Rct2 values of DSSCs with pure ZnO, pure TiO2, 3TiO2:1ZnO, 1TiO2:3ZnO, and 1TiO2:1ZnO thin films are 55.23, 24.18, 19.14, 47.17, and 33.16 Ω, respectively. It is obvious that DSSC with composite 3TiO2:1ZnO thin film depicts a smaller semicircle than other cells. The presence of smaller semicircles in this region indicates a significant decrease in load transfer resistance. The smaller Rct2 value of composite 3TiO2:1ZnO-based DSSC can be attributed to the increase in the crystallinity of the tightly packed composite nanoparticle structure, which controls the electron diffusion path length [42]. In addition, low Rct2 values can provide high power conversion efficiency by improving electrical conductivity. The lower Rct2 value in the composite 3TiO2:1ZnO-based cell was attributed to the presence of agglomerated nanoparticles that provide strong compactness between the composite-based TiO2/ZnO-dye [43]. It is clear that DSSCs with smaller charge transfer resistance have higher power conversion efficiency. This shows that the results obtained from the impedance resistance parameters and the Jsc-V characteristics are in agreement with each other.
The bode curves in Fig. 9b were used to understand the responses of a DSSC to various frequencies associated with the interfacial charge transfer mechanism. In the low frequency range, \(\tau\) and the semicircles are intimately related. The \(\tau\) value is very important for the performance of DSSCs. The following formula was used to obtain the \(\tau\) value. [44].
The highest frequency peak of the bode curves in the low frequency range is represented by fmax in Eq. 4. The \(\tau\) values of DSSCs with pure ZnO, pure TiO2, 3TiO2:1ZnO, 1TiO2:3ZnO, and 1TiO2:1ZnO thin film are 0.15, 0.66, 0.75, 0.20, and 0.24 ms, respectively. The \(\tau\) value of the cell produced with composite-based 3TiO2:1ZnO thin film is higher than other cells. This situation was attributed to the fast electron transfer and slow charge recombination in DSSCs of the close-packed structure formed by TiO2 spheres with ZnO nanorods [45]. As the ZnO ratio in cells increases, \(\tau\) values decrease, as ZnO increases the recombination rate at the electrolyte/electrode interface [5]. In addition, the electron lifetime and performance of cells support each other.
4 Conclusion
In this study, pure ZnO, Pure TiO2, 3TiO2:1ZnO, 1TiO2:3ZnO, and 1TiO2:1ZnO metal oxide nanopowders were synthesized by hydrothermal method to create photoanode thin films. XRD patterns confirmed that the synthesized TiO2 and ZnO were in anatase and hexagonal wurtzite structure, respectively. Furthermore, the absence of any additional peaks other than the characteristic peaks showed that the synthesized TiO2 and ZnO nanoparticles had high purity. SEM photographs indicated that spherical TiO2 nanoparticles tightly surrounded the ZnO nanorods, and such a structure was attributed to the increased light harvesting in 3TiO2:1ZnO-based DSSC. According to UV studies, all photoanodes exhibited absorption in the UV–vis region. However, composite 3TiO2:1ZnO thin film had stronger absorption in the visible region than other samples, and such an optical behavior was due to the new energy levels forming in the band gap of this thin film. The photovoltaic properties of DSSCs revealed that the cell with 3TiO2:1ZnO thin film had the highest power conversion performance (9.9%). The high efficiency of this DSSC was associated with the surface morphology of close-packed TiO2 nanospheres that densely surround the ZnO nanorods and the rapid electron injection. In addition, the low cell performance of composite-based DSSCs with high ZnO content was attributed to the limited overlap of excited electrons with the conduction band of ZnO. It was understood from EIS analyses that higher Rct2 values in DSSCs led to lower cell performance. The lowest Rct2 value was in the cell formed with composite 3TiO2:1ZnO photoanode. This was explained by the increase in crystallinity of the tightly packed composite nanoparticle structure controlling the electron diffusion path length.
Data availability
Not relevant to this research.
References
M. Abrari, M. Ahmadi, H.M. Chenari, M. Ghanaatshoar, Investigating the effect of ZrO2 nanofibers in ZnO-based photoanodes to increase dye-sensitized solar cells (DSSC) efficiency: inspecting the porosity and charge transfer properties in ZnO/ZrO2 nanocomposite photoanode. Opt. Mater. 147, 114690 (2024). https://doi.org/10.1016/j.optmat.2023.114690
F. Aslan, Increasing the photoelectric conversion efficiency of dye-sensitized solar cells by doping SrAl2O4:Eu+2, Dy+3 to TiO2-based photoanodes. Physica B 668, 415267 (2023). https://doi.org/10.1016/j.physb.2023.415267
E. Akman, Enhanced photovoltaic performance and stability of dye-sensitized solar cells by utilizing manganese-doped ZnO photoanode with europium compact layer. J. Mol. Liq. 317, 114223 (2020). https://doi.org/10.1016/j.molliq.2020.114223
D. Franchi, M. Bartolini, F. D’Amico, M. Calamante, L. Zani, G. Reginato, A. Mordini, A. Dessì, Exploring different designs in thieno[3,4-b]pyrazine-based dyes to enhance divergent optical properties in dye-sensitized solar cells. Processes 11, 3–16 (2023). https://doi.org/10.3390/pr11051542
M. Giannouli, Nanostructured ZnO, TiO2, and Composite ZnO/TiO2 films for application in dye-sensitized solar cells. Int. J. Photoenergy 2013, 612095 (2013). https://doi.org/10.1155/2013/612095
M.E. Yeoh, K.Y. Chan, H.Y. Wong, P.L. Low, G.S. How Thien, Z.N. Ng, H.C. Ananda Murthy, R. Balachandran, Hydrothermal duration effect on the self-assembled TiO2 photo-anode for DSSC application. Optical Mater. 141, 113907 (2023). https://doi.org/10.1016/j.optmat.2023.113907
M. Abrari, M. Ghanaatshoar, S.S. Malvajerdi, S. Gholamhosseini, A. Hosseini, H. Sun, S.M. Mohseni, Investigating various metal contacts for p-type delafossite α-CuGaO2 to fabricate ultraviolet photodetector. Sci. Rep. (2023). https://doi.org/10.1038/s41598-023-35458-0
T. Taguchi, X.T. Zhang, I. Sutanto, K.I. Tokuhiro, T.N. Rao, H. Watanabe, T. Nakamori, M. Uragami, A. Fujishima, Improving the performance of solid-state dye-sensitized solar cell using MgO-coated TiO2 nanoporous film. Chem. Commun. 3, 2480–2481 (2003). https://doi.org/10.1039/b306118c
N. Ullah, S.M. Shah, R. Ansir, S. Erten-Ela, S. Mushtaq, S. Zafar, Pyrocatechol violet sensitized cadmium and barium doped TiO2/ZnO nanostructures: as photoanode in DSSC. Mater. Sci. Semicond. Process. 135, 106119 (2021). https://doi.org/10.1016/j.mssp.2021.106119
N.G. Park, M.G. Kang, K.M. Kim, K.S. Ryu, S.H. Chang, D.K. Kim, J. Van de Lagemaat, K.D. Benkstein, A.J. Frank, Morphological and photoelectrochemical characterization of core-shell nanoparticle films for dye-sensitized solar cells: Zn-O type shell on SnO 2 and TiO 2 cores. Langmuir 20, 4246–4253 (2004). https://doi.org/10.1021/la036122x
C. Cavallo, F. Di Pascasio, A. Latini, M. Bonomo, D. Dini, Nanostructured semiconductor materials for dye-sensitized solar cells. J. Nanomater. (2017). https://doi.org/10.1155/2017/5323164
E. Akman, H.S. Karapinar, Electrochemically stable, cost-effective and facile produced selenium@activated carbon composite counter electrodes for dye-sensitized solar cells. Sol. Energy 234, 368–376 (2022). https://doi.org/10.1016/j.solener.2022.02.011
H.S. Jung, J.K. Lee, M. Nastasi, S.W. Lee, J.Y. Kim, J.S. Park, K.S. Hong, H. Shin, Preparation of nanoporous MgO-coated TiO 2 nanoparticles and their application to the electrode of dye-sensitized solar cells. Langmuir 21, 10332–10335 (2005). https://doi.org/10.1021/la051807d
S.P. Lim, Y.S. Lim, A. Pandikumar, H.N. Lim, Y.H. Ng, R. Ramaraj, D.C.S. Bien, O.K. Abou-Zied, N.M. Huang, Gold-silver@TiO2 nanocomposite-modified plasmonic photoanodes for higher efficiency dye-sensitized solar cells. Phys. Chem. Chem. Phys. 19, 1395–1407 (2017). https://doi.org/10.1039/c6cp05950c
S.S. Kanmani, K. Ramachandran, Synthesis and characterization of TiO 2/ZnO core/shell nanomaterials for solar cell applications. Renew. Energy 43, 149–156 (2012). https://doi.org/10.1016/j.renene.2011.12.014
S. Suhaimi, M.M. Shahimin, Z.A. Alahmed, J. Chyský, A.H. Reshak, Materials for enhanced dye-sensitized solar cell performance: Electrochemical application. Int. J. Electrochem. Sci. 10, 2859–2871 (2015). https://doi.org/10.1016/s1452-3981(23)06503-3
N. Sakai, T. Miyasaka, T.N. Murakami, Efficiency enhancement of ZnO-based dye-sensitized solar cells by low-temperature TiCl4 treatment and dye optimization. J. Phys. Chem. C 117, 10949–10956 (2013). https://doi.org/10.1021/jp401106u
R. Zhao, L. Zhu, F. Cai, Z. Yang, X. Gu, J. Huang, L. Cao, ZnO/TiO2 core-shell nanowire arrays for enhanced dye-sensitized solar cell efficiency. Appl. Phys. A Mater. Sci. Process. 113, 67–73 (2013). https://doi.org/10.1007/s00339-013-7663-x
K.M. Lee, E.S. Lee, B. Yoo, D.H. Shin, Synthesis of ZnO-decorated TiO2nanotubes for dye-sensitized solarcells. Electrochim. Acta 109, 181–186 (2013). https://doi.org/10.1016/j.electacta.2013.07.055
Y.L. Xie, Z.X. Li, Z.G. Xu, H.L. Zhang, Preparation of coaxial TiO2/ZnO nanotube arrays for high-efficiency photo-energy conversion applications. Electrochem. Commun. 13, 788–791 (2011). https://doi.org/10.1016/j.elecom.2011.05.003
A.G. Thate, K.S. Pakhare, S.S. Patil, V.M. Bhuse, Fabrication of TiO2-ZnO nanocomposite photoanodes to enhance the dye-sensitized solar cell efficiency. Res. Chem. Intermed. 49, 147–168 (2023). https://doi.org/10.1007/s11164-022-04878-4
B.A. Kumar, V. Vetrivelan, G. Ramalingam, A. Manikandan, S. Viswanathan, P. Boomi, G. Ravi, Computational studies and experimental fabrication of DSSC device assembly on 2D-layered TiO2 and MoS2@TiO2 nanomaterials. Physica B: Condens. Matter. (2022). https://doi.org/10.1016/j.physb.2022.413770
G. Nagaraj, D. Brundha, C. Chandraleka, M. Arulpriya, V. Kowsalya, S. Sangavi, R. Jayalakshmi, S. Tamilarasu, R. Murugan, Facile synthesis of improved anatase TiO2 nanoparticles for enhanced solar-light driven photocatalyst. SN Appl. Sci. (2020). https://doi.org/10.1007/s42452-020-2554-1
M. Achehboune, M. Khenfouch, I. Boukhoubza, B. Mothudi, I. Zorkani, A. Jorio, Study on the effect of Er dopant on the structural properties of ZnO nanorods synthesized via hydrothermal method. J. Phy. Conf Ser. (2019). https://doi.org/10.1088/1742-6596/1292/1/012020
M. Sufyan, U. Mehmood, Y. Qayyum Gill, R. Nazar, A. Ul Haq Khan, Hydrothermally synthesize zinc oxide (ZnO) nanorods as an effective photoanode material for third-generation dye-sensitized solar cells (DSSCs). Mater. Lett. 297, 130017 (2021). https://doi.org/10.1016/j.matlet.2021.130017
Z. Zheng, W. Xie, Z.S. Lim, L. You, J. Wang, CdS sensitized 3D hierarchical TiO2/ZnO heterostructure for efficient solar energy conversion. Sci. Rep. 4, 1–6 (2014). https://doi.org/10.1038/srep05721
A. Maru, C.M. Panchasara, K.N. Rathod, H. Boricha, K. Sagapariya, B. Udeshi, A. Vaishnani, A.D. Joshi, D.D. Pandya, P.S. Solanki, N.A. Shah, UV–Visible spectroscopy and dielectric studies of ZnO/TiO2 composite. Bulletin Mater. Sci. (2023). https://doi.org/10.1007/s12034-023-02985-5
S.K. Dhungel, J.G. Park, Optimization of paste formulation for TiO2 nanoparticles with wide range of size distribution for its application in dye sensitized solar cells. Renew. Energy 35, 2776–2780 (2010). https://doi.org/10.1016/j.renene.2010.04.031
H. Farheen, S.G. Fouad, A.A. Ansari, Z.A. Khan, Ansari, europium doped TiO2: an efficient photoanode material for dye sensitized solar cell. J. Mater. Sci.: Mater. Electron. 28, 6873–6879 (2017). https://doi.org/10.1007/s10854-017-6387-7
M. Saeidi, M. Abrari, M. Ahmadi, Fabrication of dye-sensitized solar cell based on mixed tin and zinc oxide nanoparticles. Appl. Phys. A Mater. Sci. Process. 125, 1–9 (2019). https://doi.org/10.1007/s00339-019-2697-3
N. Dhananjaya, H. Nagabhushana, B.M. Nagabhushana, B. Rudraswamy, S.C. Sharma, D.V. Sunitha, C. Shivakumara, R.P.S. Chakradhar, Effect of different fuels on structural, thermo and photoluminescent properties of Gd2O3 nanoparticles, Spectrochimica Acta—Part A: molecular and biomolecular. Spectroscopy 96, 532–540 (2012). https://doi.org/10.1016/j.saa.2012.04.067
R. Syafinar, N. Gomesh, M. Irwanto, M. Fareq, Y.M. Irwan, Chlorophyll pigments as nature based dye for dye-sensitized solar cell (DSSC). Energy Procedia (2015). https://doi.org/10.1016/j.egypro.2015.11.584
T.-C. Li, T.-H. Kuan, J.-F. Lin, Effects of inclination angle during Al-doped ZnO film deposition and number of bending cycles on electrical, piezoelectric, optical, and mechanical properties and fatigue life. J. Vac. Sci. Technol. Vac. Surf. Films. (2016). https://doi.org/10.1116/1.4936093
H.D. Jang, S.K. Kim, H. Chang, K.M. Roh, J.W. Choi, J. Huang, A glucose biosensor based on TiO2-Graphene composite. Biosens. Bioelectron. 38, 184–188 (2012). https://doi.org/10.1016/j.bios.2012.05.033
F. Aslan, New natural dyes extracted by ultrasonic and soxhlet method: effect on dye-sensitized solar cell photovoltaic performance, optical and quantum. Electronics 56, 1–22 (2024). https://doi.org/10.1007/s11082-024-06294-x
J. Cen, Q. Wu, D. Yan, W. Zhang, Y. Zhao, X. Tong, M. Liu, A. Orlov, New aspects of improving the performance of WO 3 thin films for photoelectrochemical water splitting by tuning the ultrathin depletion region. RSC Adv. 9, 899–905 (2019). https://doi.org/10.1039/c8ra08875f
N. Wang, D. Wang, M. Li, J. Shi, C. Li, Photoelectrochemical water oxidation on photoanodes fabricated with hexagonal nanoflower and nanoblock WO3. Nanoscale 6, 2061–2066 (2014). https://doi.org/10.1039/c3nr05601e
A. Elkhidir Suliman, Y. Tang, L. Xu, Preparation of ZnO nanoparticles and nanosheets and their application to dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 91, 1658–1662 (2007). https://doi.org/10.1016/j.solmat.2007.05.014
N.D. Desai, K.V. Khot, T. Dongale, K.P. Musselman, P.N. Bhosale, Development of dye sensitized TiO2 thin films for efficient energy harvesting. J. Alloy. Compd. 790, 1001–1013 (2019). https://doi.org/10.1016/j.jallcom.2019.03.246
S. Sigdel, H. Elbohy, J. Gong, N. Adhikari, K. Sumathy, H. Qiao, Q. Wei, M.H. Sayyad, J. Zai, X. Qian, Q. Qiao, Dye-sensitized solar cells based on porous hollow tin oxide nanofibers. IEEE Trans. Electron Devices 62, 2027–2032 (2015). https://doi.org/10.1109/TED.2015.2421475
S.S. Patil, R.M. Mane, K.V. Khot, S.S. Mali, C. Kook Hong, P.N. Bhosale, Surfactant assisted approach to development of efficient WO3 photoanode for natural dye sensitized solar cell. Sol. Energy. 220, 371–383 (2021). https://doi.org/10.1016/j.solener.2021.03.026
J. Chu, D. Lu, X. Wang, X. Wang, S. Xiong, WO3nanoflower coated with graphene nanosheet: synergetic energy storage composite electrode for supercapacitor application. J. Alloy. Compd. 702, 568–572 (2017). https://doi.org/10.1016/j.jallcom.2017.01.226
R. Kishore, X. Cao, X. Zhang, A. Bieberle-Hütter, Electrochemical water oxidation on WO3 surfaces: a density functional theory study. Catal. Today 321–322, 94–99 (2019). https://doi.org/10.1016/j.cattod.2018.02.030
R. Kern, R. Sastrawan, J. Ferber, R. Stangl, J. Luther, Modeling and interpretation of electrical impedance spectra of dye solar cells operated under open-circuit conditions. Electrochim. Acta 47, 4213–4225 (2002)
M.G. Aswani Yella, H.W. Lee, H.N. Tsao, C. Yi, A.K. Chandiran, Md. Khaja Nazeeruddin, E. W.G. Diau, C.Y. Yeh, S.M. Zakeeruddin, This faster interfacial charge recombPorphyrin-sensitized solar cells with yield shorter electron lifetimes when used in the DSCina- Cobalt (II/III)–based redox electrolyte tion, when compared with iodide/triiodide–based redox electrolytes (12–18), lowe. Science. 334, 629–633 (2011)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by TEKF.20.24 Fırat University Scientific Research Projects Unit (FÜBAP). We would like to thank the FÜBAP unit for their support.
Author information
Authors and Affiliations
Contributions
F.A contributed toward experimental studies, scientific calculation, and writing. H.E contributed toward methodology and research. A.D contributed toward resources and edits the original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this paper disclose that they have no competing interests.
Ethical approval
Not relevant to this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Demir, A., Aslan, F. & Esen, H. TiO2/ZnO-based composite thin films coated on FTO surface by screen printing method: increasing dye-sensitized solar cell performance. J Mater Sci: Mater Electron 35, 1481 (2024). https://doi.org/10.1007/s10854-024-13213-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-13213-z