Abstract
In this investigation, we synthesized nano-sized perovskite LSMO and Cu-doped LSMCx materials using the sol–gel method, exhibiting appropriate Curie temperatures and magnetic attributes conducive to the application of self-controlled hyperthermia. Centrifugal separation has been used to reduce particle size distribution and thus analyze magnetic properties dependent on mean particle size. Structural analysis was conducted using X-ray Powder Diffraction. The composition was determined through X-ray Photoelectron Spectroscopy, while topographical features were scrutinized employing Scanning Electron Microscopy. Magnetic properties were evaluated employing a Vibrating Sample Magnetometer, and the magneto-thermal characteristics were delineated using an Alternating Magnetic Field hyperthermia system. Notably, this study marks the pioneering identification of La1−xSrxMnO3 as a viable material candidate for auto-regulated hyperthermia, based on its magnetization range (5–20 emu/g) and Curie temperature span (287–357 K) that are changing with the mean particle size. Through comprehensive analysis, we thoroughly investigated its hyperthermia attributes, thereby contributing significant insights to the existing literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer, a malady marked by its escalating incidence, persists as a formidable challenge in the modern medical landscape [1]. Despite earnest efforts over recent years to innovate diagnostic and therapeutic methodologies, cancer continues to be a principal global cause of mortality, its complex and costly treatment regimen highlighting its persistence as a leading cause of death [2, 3]. The spectrum of contemporary cancer treatment encompasses various modalities, including chemotherapy, radiotherapy, and surgery, with surgical interventions also serving diagnostic purposes. Chemotherapy and radiotherapy, commonly employed post-surgery or in instances where surgical intervention is unfeasible, suffer from their inability to discriminate effectively between cancerous and healthy tissues, resulting in collateral damage to the latter and a compromised patient quality of life during treatment.
This backdrop has spurred the exploration of alternative treatment strategies, emphasizing localized targeting. While drug targeting holds promise, the synthesis of chemotherapeutic agents for this approach remains both intricate and expensive, compounded by the necessity for custom formulations for each cancer type. A promising avenue in this context is hyperthermia, an approach utilizing cost-efficient inorganic magnetic nanomaterials that transcend cancer type restrictions. This method involves raising the temperature of the body or tissue to the therapeutic range of approximately 42–45 °C, denaturing cancer cell enzymes that are rendered more susceptible due to irregular blood circulation networks and limited cooling mechanisms, characteristics less prevalent in healthy tissues [4]. This method’s conceptual foundation traces back to the seminal work of German surgeon Carl D.W. Busch in 1866, which showcased the potential of elevated temperatures to selectively target and eradicate cancer cells without harming healthy ones [5]. Subsequent research advancements accelerated the deployment of clinical hyperthermia during the 1980s, marking a departure from whole-body heating to focus on intensive targeted approaches [6].
While hyperthermia demonstrates various advantages, it also harbors inherent drawbacks necessitating resolution. To surmount these challenges, the research community has directed efforts towards developing methods that are noninvasive, exhibit minimal or zero toxicity, and surpass the efficacy of existing paradigms [7,8,9]. Among these efforts, magnetic hyperthermia emerged as a concept in 1957, gaining increasing traction in subsequent years. Gilchrist et al. introduced the notion of magnetic hyperthermia through the injection of magnetic Fe2O3 particles (of size 20–100 nm) into lymph nodes, subsequently applying variable magnetic fields (AMF) to target lymphatic metastases [10]. Initial magnetic hyperthermia trials utilizing magnetic microseeds faced limitations akin to traditional hyperthermia, including the requirement for surgical implantation and challenges in treating deeply seated tumors. The innovation of Magnetic Nanofluid Hyperthermia (MNH) or Magnetic Hyperthermia (MHT) harnessed magnetic nanoparticles (MNPs) stably suspended in colloidal fluids like water or hydrocarbons as therapeutic agents, alleviating previous obstacles [11, 12]. This shift paved the way for MNPs to emerge as a promising class of materials for effective cancer diagnosis and treatment, offering diverse biocompatible properties, often modulatable through external magnetic fields.
Central to the effectiveness of MNPs in biomedical applications are factors such as appropriate size, optimal magnetic properties, water solubility, biocompatibility, and surface coatings that ensure stability, selectivity, and protection against aggregation or oxidation. The distinctiveness of MNPs lies in their potential to detect incipient cancer stages and serve as intermediaries in localized cancer treatment through hyperthermia. Their functionalized surfaces can specifically target rare cancer cells undetectable by conventional imaging, offering a breakthrough in early-stage diagnosis. Additionally, MNPs can be co-administered with anticancer drugs to achieve simultaneous diagnosis and treatment, enhancing accuracy and efficacy. In this regard, MNPs hold promise as multifunctional theranostic agents, playing roles in diagnosis, MRI contrast enhancement, hyperthermia treatment, and controlled drug delivery. However, the diverse applications of MNPs mandate precise determination of their physical and chemical properties, which significantly influence their performance in different contexts, including biocompatibility and cytotoxicity.
Notably, the focus of this study revolves around La1−xSrxMnO3 (LSMO) perovskite nanoparticles synthesized via the Sol–gel Pechini method, offering a multifaceted platform for hyperthermia treatment. LSMO emerges as an attractive candidate due to its tunable Curie temperature (Tc) that allows for temperature-controlled hyperthermia treatment without resorting to variable external magnetic fields [13, 14]. This self-regulated magnetic hyperthermia holds promise for effective cancer treatment. Despite the existing body of research on MNPs, comprehensive studies on perovskite-structured, especially LSMO-structured, MNPs within the hyperthermia field remain scarce, warranting exploration.
Herein, we present a comprehensive investigation into the synthesis, characterization, and applicability of LSMO nanoparticles synthesized via the Sol–gel Pechini method. The ensuing sections delve into the structural, compositional, topographical, magnetic, and magneto-thermal attributes of these nanoparticles, shedding light on their potential as a key player in the realm of hyperthermia-based cancer treatment.
2 Experimental
For the aims of the study, perovskite structures of La0.7Sr0.3MnO3 (LSMO) and La0.7Sr0.3Mn1−xCuxO3 (LSMCx; x = 0.1, 0.2) compositions, incorporating varying Cu doping ratios, were synthesized using the sol–gel method [15, 16]. As starting materials, high-purity La(NO3)3·6H2O (99 + % purity; Chempur, Germany), Cu(NO3)2·3H2O (99 + % purity; Sigma Aldrich, USA), Manganese (II) acetate (98 + % purity; Sigma Aldrich, USA) and Sr(NO3)2 (98 + % purity; Sigma Aldrich, USA) were used according to stoichiometry. The resulting LSMC0 (x = 0) sample was further fractionated into sub-particle distribution groups using centrifugation at distinct speeds, thereby obtaining samples with different average particle sizes.
Structural analysis was conducted via X-ray powder diffraction (XRD) measurements using a Rigaku D-Max/B powder diffraction instrument equipped with characteristic Cu-Kα X-ray radiation (λ = 0.154 nm). Scans were performed within the 2θ range of 20–80° at an increment of ∆(2θ) = 0.02°. XPS spectrometry was employed to ascertain the composition ratios of the doped samples, validating the attainment of the anticipated chemical composition.
Magnetic properties were characterized using the Physical Properties Measuring System (PPMS) with VSM (Vibrating Sample Magnetometer) option. Low-temperature magnetization (M–T) measurements were executed within the temperature range of 10–340 K under an applied field of 500 Oe. Additionally, high-temperature measurements spanning 310–450 K were conducted under the same field conditions. Magnetization measurements in response to external magnetic fields were captured over a ± 30 kOe range.
Magneto-thermal behavior was assessed through a custom magneto-thermal measurement system employing an RF signal generator and a fiber optic temperature sensor. Measurements were taken with a variable magnetic field frequency of 300 kHz and power of 160 W, following stable dispersion of samples in ethanol (1.3 mg sample/0.5 mL ethanol). To mitigate heat loss to the environment, the glass tube containing the sample was insulated, ensuring heat generation was confined to the nanoparticle medium. This approach assumed negligible temperature changes in the solution [17].
Given the influence of particle size on Curie temperature, efforts were directed at reducing particle size distribution to achieve a well-defined Curie transition for self-controlled hyperthermia applications [18]. The LSMC0 sample was sonicated in ethyl alcohol and precipitated through centrifugation at increasing speeds (500, 1000, 1500, 2500, 5000, 10,000 rpm) to create samples with progressively smaller particle sizes. Literature insight guided this process, as larger particles were expected to sediment at lower speeds.
The described experimental procedures provide a comprehensive framework for the synthesis, characterization, and preparation of samples, vital to understanding the subsequent analysis of perovskite structures and their potential application in self-controlled hyperthermia treatments.
3 Results and discussion
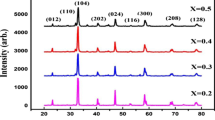
The X-ray diffraction (XRD) patterns of the LSMCx samples unequivocally demonstrate their single-phase nature, as depicted in Fig. 1. Notably, there is a discernible trend wherein the principal peak undergoes a systematic shift towards larger diffraction angles in direct correlation with the doping of Cu ions. This observable phenomenon is attributed to the substitution of Mn ions by Cu ions, the latter possessing a smaller ionic radius. This substitution is indicative of Cu atoms’ incorporation within the crystalline lattice in lieu of Mn.
Refinement of the XRD results were made using MaudLab program, and the results for LSMC0 sample are shown in Fig. 1. As shown in Table 1, calculations exhibit a general diminishing trend with doping ratio. Specifically, the particle size diminishes successively from 97.6 nm for the pristine composition to 96.2 nm and subsequently to 87.2 nm with increasing Cu admixture. Meanwhile, isotropic micro-strain decreases from 0.0078 to 0.0033. Lattice parameters also show a trend by the increase of Cu admixture. While a increases, b and c decrease because of the smaller ionic radius of Cu than Mn.
Analysis of the XPS measurement results of doped LSMCx samples using the advantage program shows that the Cu/Mn atom ratio of the samples was realized in accordance with the stoichiometric predictions (Table 2).
The magnetic properties of the LSMCx samples were characterized using Magnetization–Temperature (M–T) and Magnetization–Field (M–H) curves obtained via Vibrating Sample Magnetometry (VSM). These analyses reveal that the samples exhibit superparamagnetic behavior at temperatures proximal to room temperature, transitioning into a paramagnetic state at temperatures exceeding room temperature. This aligns precisely with the anticipated outcome, indicating a Curie temperature in close proximity to ambient conditions [19].
Specifically, the undoped sample displays a remarkably sharp transition, as depicted in Fig. 2a. In contrast, a discernible absence of such a sharp transition is observed in the doped samples. This discrepancy may be attributed to an unexpectedly broader particle size distribution stemming from the employed production methodology.
a Normalized magnetization (M–T) curves of LSMCx samples, measured between 10 and 500 K (both low and high temperatures), b The linear coupling operation (red line) performed on the linear part of the ZFC curve of the M–T curve obtained for the LSMC10 sample as an example, and the parameters for the linear function (table)
The Curie temperature values, as exemplified in Fig. 2b and summarized in Table 3, corroborate this observation. Evidently, an increase in the Cu doping ratio leads to a systematic reduction in Curie temperature. Additionally, the blocking temperatures of the samples show a declining trend, commencing at 130 K and progressively decreasing to 90 K and 70 K, respectively, with increasing Cu ratio.
For superparamagnetic materials, it is established that the energy of anisotropy at the blocking temperature equates to the thermal energy, as expressed by Eq. 1.
Here, k represents the Boltzmann constant, Ket signifies the effective anisotropy constant, and V denotes the particle volume. In this context, assuming a spherical particle geometry in samples prepared via sol–gel synthesis, the calculated anisotropy coefficient derived from Eq. 1 stands at 3.87 J/m3 for the undoped sample. This value experiences a gradual reduction to 3.48 J/m3 and 3.35 J/m3, respectively, with increasing doping ratios (see Table 3).
The diminishing anisotropy with increasing doping levels is indicative of a concurrent decrease in spin-orbital interactions. This reduction, in turn, contributes to the observed decline in Curie temperature. Importantly, this finding is consistent with the alterations in Curie temperatures discerned from M–T measurements, reinforcing the pivotal role of doping in modulating the magnetic behavior of the LSMCx samples.
As can be seen from the room temperature M–H measurements given in Fig. 3, the results support that the samples are superparamagnetic.
The Magnetization–Temperature (M–T) curves for LSMC0 samples, characterized with varying average particle sizes achieved through centrifugation, were scrutinized over a temperature range spanning from 10 to 500 K, encompassing both low and high-temperature regimes (Fig. 4). Intriguingly, it became evident that the Curie temperature exhibited notable variation contingent on the applied centrifuge speed.
To discern and quantify this effect, linear regression analyses were conducted on the M–T curves, yielding the Curie temperature values for each sample (Fig. 5). These values, pivotal to the understanding of the magnetic behavior, are comprehensively presented in Table 4. This systematic analysis underscores the significant influence of particle size manipulation through centrifugation on the magnetic properties of the LSMC0 samples.
The Magnetization–Field (M–H) curves, acquired at ambient temperature, provide further insight into the magnetic properties of the samples under investigation (Fig. 6). Remarkably, the data unequivocally demonstrate that all samples exhibit superparamagnetic behavior at room temperature, affirming their characteristic response to an applied magnetic field.
Notably, it is discerned that the sample subjected to high-speed centrifugation at 5000 rpm manifests a notably higher saturation magnetization in comparison to its counterparts. This observation finds its explanation in the reduction of particle size and the concurrent narrowing of the size distribution range. Literature affirms and supports this phenomenon through a body of both experimental and modeling studies [20, 21]. Such investigations collectively underscore the pivotal role of controlled particle size manipulation in tailoring the magnetic characteristics of materials, a principle profoundly exemplified in our experimental findings.
The magnetic measurements conducted indicate that the Curie temperature of the produced samples was found to be above room temperature, closely aligning with the treatment temperature. Consequently, Scanning Electron Microscopy (SEM) was employed to obtain precise average particle size measurements for the centrifugally separated samples. Subsequently, hyperthermia performance and the associated cut-off temperature variations were evaluated in relation to the average particle size through dedicated hyperthermia measurements conducted on these samples.
Following centrifugation-based separation, it was observed that the magnetic nanoparticles didn’t exhibit a tendency to precipitate within a span of time more than 10 min, subsequent to ultrasonic probe dispersion in ethanol. To ensure consistency, the dispersion ratio for all samples was maintained at 2.6 g/L. Thereafter, the magneto-thermal properties of these dispersions were probed using an induction furnace. For these assessments, 0.5 mL of the dispersion was introduced into a coil generating a variable magnetic field, operating at 160 W power and a frequency of 300 kHz. Subsequent temperature changes were recorded over time using a fiber-optic thermometer. The resultant data is illustrated in Fig. 7 for LSMCx samples, and Fig. 8 for centrifugally separated LSMC0 samples.
In the Temperature Difference vs. Time (DeltaT-t) plots, commencing from room temperature, the samples exhibited distinct temperature elevations. Intriguingly, it was noted that each sample ceased heating upon attaining a specific temperature threshold, exhibiting varying degrees of temperature change, until this cut-off temperature was attained. Given the uniformity in sample preparation, solvent/solute ratio, and the consistent application of an external magnetic field with identical parameters, a comparative analysis of the magneto-thermal behavior is justified. In line with the anticipated trends from the Curie temperatures detailed in Table 3, the LSMC0 sample displayed more pronounced temperature increments compared to other doped samples, with the doped variants demonstrating less substantial temperature changes (Fig. 7). These observations provide valuable insights into the nuanced hyperthermic properties of the examined samples.
The Temperature Difference vs. Time (DeltaT-t) measurements for the centrifugally separated samples notably highlighted the distinctive heating behavior, particularly evident in the sample precipitated at 2500 rpm, which exhibited a remarkably elevated thermal response compared to the others (Fig. 8, Table 5).
It is noteworthy that in both LSMCx and centrifugally separated LSMC0 samples, the attained temperatures did not align with the Curie temperatures delineated in Tables 3 and 4, as observed during the magnetic hyperthermia process (Figs. 7 and 8). This discrepancy finds explanation in the observation that the phase transition at the Curie temperature lacks the requisite sharpness to induce the anticipated thermal response. This nuance underscores the intricate nature of the magnetic properties and the need for a nuanced understanding of the phase transition dynamics in these materials for effective hyperthermia applications.
Hyperthermia measurements showed that saturation temperatures were lower than the calculated Curie temperatures. The reason for this is thought to be the process by which the experimental system, although isolated, returns to thermal equilibrium with the environment, which is kept constant at 21 °C throughout the experiments.
The heating rate in magnetic hyperthermia applications is subject to various influencing factors, including the quantity of magnetic nanoparticles and, consequently, their density, as well as the properties of the surrounding medium. To facilitate meaningful comparisons with other magnetic materials in hyperthermia applications, it is imperative to normalize heating rate values relative to the amount of material and the heat capacity of the medium [22].
The quantitative representation of thermal power harnessed through Magnetic Nanoparticle (MNP) exposure to a variable magnetic field, akin to a power density metric, is encapsulated by the Specific Absorption Rate (SAR). This is defined by Eq. 2:
where C denotes the heat capacity of the nanofluid, ms represents the mass of the nanofluid, mm signifies the mass of the magnetic material, and \(\frac{\Delta T}{\Delta t}\) corresponds to the slope derived from the linear segment of the temperature–time graph [23]. The SAR values for the samples were computed using Eq. 2, leveraging the slopes of the linear segments from the DeltaT-t graphs, one sample of which is illustrated as an example in Figs. 9 and 10.
Implicit in this calculation was the assumption of a constant specific heat for the solvent medium. The incorporation of copper (Cu) through doping aimed to lower the Curie temperature, bringing it closer to room temperature. However, this endeavor resulted in Curie temperatures falling below room temperature. As delineated in Table 5, it was evident that samples, excluding LSMC0, yielded inadequate heating, with correspondingly minuscule SAR values. LSMC0 samples, which exhibited high SAR calculations, were further stratified based on sub-particle distribution groups achieved through centrifugation. This stratification yielded accelerated heating as anticipated (Fig. 8), ultimately resulting in higher evaluated SAR values (Table 5). Notably, the observation that the sample centrifuged at 1000 rpm, with a predicted larger average particle size, exhibited the smallest SAR value, underscores the potential for enhanced heating rates through particle size reduction. This insight offers crucial implications for tailoring hyperthermic performance in magnetic materials.
The calculated SAR values of all samples are given comparatively in Fig. 11. Accordingly, both the LSMC0 sample and the size-separated LSMC0 samples by centrifuge appear to have higher SAR values than the LSMC10 and LSMC20 samples. In addition, the results show that the sample of LSMC0 separated by centrifugation at 2500 rpm has the highest values.
When the mean particle sizes of the centrifuged samples were calculated using the SEM images taken for centrifugal size-separated subgroups of the LSMC0 sample (Fig. 12), it was seen that the average particle size decreased with the increase in centrifuge speed, as expected. As can be seen in Table 6 and Fig. 13, which were prepared as a result of the analysis of the effect of particle size on the SAR value, in general, faster heating and effective hyperthermia were observed in small particle size materials.
The observed similarity in Specific Absorption Rate (SAR) values between the samples investigated and those reported in existing literature underscores the potential significance of these materials in magneto-thermal applications. While SAR values can vary depending on factors such as production methodology, environmental conditions, and the intensity/frequency of the applied variable magnetic field, a substantial body of research has consistently demonstrated SAR values within the range of 30–70 W/g. Noteworthy examples include an SAR of 55 W/g obtained from a hyperthermia study employing maghemite nanoparticles with an average size of 14 nm dissolved in water, and an SAR of 41.9 W/g achieved through a hyperthermia study utilizing Fe3O4 nanoparticles with an average size of 11.7 nm dissolved in isoparaffin [22, 24]. These instances serve as notable benchmarks in the field.
Moreover, the literature suggests that specific size ranges may be particularly conducive to achieving high SAR values for certain samples [25]. The presence and variability of cut-off temperatures in our study further indicate that these materials offer viable prospects for controlled self-regulating hyperthermia, contingent on tailored atomic compositions within the material structure. This observation highlights a potential avenue for precise control and optimization of hyperthermic applications, with implications for targeted therapeutic interventions.
4 Conclusion
In conclusion, the utilization of magnetic nanoparticles for hyperthermia presents a pivotal avenue in combatting diseases through the targeted disruption of tumor cells within an alternating current (AC) magnetic field. The integration of magnetic nanoparticles in medical and biotechnological domains, particularly in hyperthermia and drug delivery systems, constitutes a focal point in contemporary health and life sciences research. Extensive investigations have been dedicated to synthesizing magnetic materials tailored for this purpose, with a specific emphasis on controlling their structural and magnetic attributes, notably particle size.
Among the various types of MNPs with low Tc to be used in magnetic hyperthermia applications, LSMO type perovskite oxides are reported to be one of the particular interests as a self-controlled heating agent with wide range of Tc between 20 and 90 °C which can be tuned by varying Sr-doping level [26, 27] and rather easy synthesis methods allowing size modification [28].
In light of this study’s findings, it is evident that La0.7Sr0.3MnO3 and La0.7Sr0.3Mn1−xCuxO3 samples stand as pivotal and promising realms of inquiry and application. Their potential for self-regulated magnetic hyperthermia applications is underscored by the judicious adjustment of Sr and Cu additive ratios, leading to the attainment of nanoparticles with narrow size distributions. These advancements, facilitated through refinements in the sol–gel production pathway, illuminate a path towards more precise and effective hyperthermic interventions, holding substantial promise for the future of therapeutic modalities in oncology and related fields.
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
References
Natl. Cancer Inst. (y.y.). https://www.cancer.gov/about-cancer/understanding/statistics. Accessed 15 July 2023
F. Bray, J. Ferlay, I. Soerjomataram, CA A Cancer J. Clin. 68, 394–424 (2018)
P. Grodzinski, M. Kircher, M. Goldberg, A. Gabizon (2019). https://doi.org/10.1021/acsnano.9b04266
L.J. Anghileri, J. Robert, Hyperthermia in Cancer Treatment: Volume 2 (CRC Press, Boca Raton, 2019)
C.D.W. Busch, Verhandl naturn Preuss Rein Westphal 23, 28 (1866)
Ç.E.D. Dönmez, A. Dönmez İçinde, in Acad. Stud. Sci. Math. Sci., ed. by T. Ucuncu. Sema Isisag; Soldatovic (Livre de Lyon, Lyon, 2020), ss. 13–32
T. Kobayashi, Biotechnol. J. 6, 1342 (2011)
S. Dutz, R. Hergt, Nanotechnology 25, 452001 (2014)
X.Y. Wong, A. Sena-Torralba, R. Álvarez-Diduk, K. Muthoosamy, A. Merkoçi, ACS Nano. 14, 2585 (2020)
R.K. Gilchrist, R. Medal, W.D. Shorey, R.C. Hanselman, J.C. Parrott, C.B. Taylor, Ann. Surg. 146, 596 (1957)
S. Dutz, R. Hergt, Int. J. Hyperth. 29, 790 (2013)
Z. Hedayatnasab, F. Abnisa, W.M.A.W. Daud, Mater. Des. (2017). https://doi.org/10.1016/j.matdes.2017.03.036
C.S.S.R. Kumar, F. Mohammad, Adv. Drug Deliv. Rev. 63, 789 (2011)
P. Thamilmaran, M. Arunachalam, S. Sankarrajan, K. Sakthipandi, E.J.J. Samuel, M. Sivabharathy, J. Magn. Magn. Mater. 443, 29 (2017)
M. Zarbali, A. Göktaş, I.H. Mutlu, S. Kazan, A.G. Şale, F. Mikailzade, J. Supercond. Nov. Magn. 25, 2767 (2012)
Q. Ren, Y. Zhang, Y. Chen, G. Wang, X. Dong, X. Tang, J. Sol-Gel Sci. Technol. 67, 170 (2013)
I. Andreu, E. Natividad, Int. J. Hyperth. 29, 739 (2013)
A.V. Pashchenko, N.A. Liedienov, I.V. Fesych, Q. Li, V.G. Pitsyuga, V.A. Turchenko, V.G. Pogrebnyak, B. Liu, G.G. Levchenko, RSC Adv. 10, 30907 (2020)
M. Phan, S. Yu, J. Magn. Magn. Mater. 308, 325 (2007)
Q. Jiang, X. Cui, M. Zhao, Appl. Phys. A 78, 703 (2004)
M. Vigneswari, K. Sakthipandi, S. Sankarrajan, IJERT Int. J. Eng. Res. Technol. 3, 13 (2014)
P. De Presa, Y. Luengo, M. Multigner, R. Costo, M. P. Morales, G. Rivero, A. Hernando (2012). https://doi.org/10.1021/jp310771p
Ö. Çelik, Kanser Tedavisinde Kullanılabilecek Magnetik Nanoparçacıkların Üretilmesi, Hacettepe Üniversitesi (2010)
G. Vallejo-Fernandez, O. Whear, A.G. Roca, S. Hussain, J. Timmis, V. Patel, K. O’Grady, J. Phys. D. Appl. Phys. 46, 312001 (2013)
Z. Li, M. Kawashita, N. Araki, M. Mitsumori, M. Hiraoka, M. Doi, Mater. Sci. Eng. C 30, 990 (2010)
M. Soleymani, M. Edrissi, A.M. Alizadeh, J. Mater. Chem. B 5, 4705 (2017)
H. Das, A. Inukai, N. Debnath, T. Kawaguchi, N. Sakamoto, S.M. Hoque, H. Aono, K. Shinozaki, H. Suzuki, N. Wakiya, J. Phys. Chem. Solids 112, 179 (2018)
N.H. Nam, D.T.M. Huong, N.H. Luong, IEEE Trans. Magn. 50, 18 (2014)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Enis SERT, Mehmet Burak KAYNAR and Şadan ÖZCAN. The first draft of the manuscript was written by Enis SERT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sert, E., Kaynar, M.B. & Özcan, Ş. Study of La1−xSrxMnO3 nanoparticles: synthesis, magnetic properties and their hyperthermia applications. J Mater Sci: Mater Electron 35, 1221 (2024). https://doi.org/10.1007/s10854-024-12977-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12977-8