Abstract
Multi-oxide photocatalytic materials derived from metal organic frameworks (MOFs) are attracting widespread attention in recent years. The heterostructure originating from oxide–oxide junctions and nonmetal doping due to the combustion of organics from MOFs provide desirable properties to the photocatalytic material. In this study, the effects of annealing temperature on the material properties and photocatalytic activity of ZIF-8-derived ZnO/TiO2 thin films were investigated. XRD, XPS, and FTIR analyses confirmed the oxidation of ZIF-8 to ZnO and SEM analysis revealed the formation of a porous structure at the annealing temperature of 500 °C. UV–Vis analysis indicated that the band gap energy decreased from 3.23 to 2.04 eV with increasing annealing temperature. Photocatalytic methylene blue degradation experiments showed that the thin film annealed at 500 °C had higher activity than the films annealed at lower temperatures. The thin film annealed at 500 °C had more than two times the dye degradation efficiency of the film annealed at 400 °C (72% and 33%, respectively). This film was also active in the photocatalytic degradation of caffeic acid which is a colorless pollutant. The high photocatalytic activity of the thin film annealed at higher temperature was attributed to its narrower band gap energy, higher porosity, and more efficient electron–hole separation, which are due to the presence of heterostructure, nonmetal doping, and deficient zones formed by the oxidation of imidazole rings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The design and production of innovative hybrid materials play a key role in solving many environmental related problems that we are facing today. It is important to produce new materials with novel nanotechnological approaches to eliminate water and air pollution, create clean energy sources with cheap and easily applicable methods. Photocatalysis can be used in the creation of sustainable and environmentally friendly processes since it uses solar energy as energy input which is free and abundant. The key step in maximizing the efficiency of the utilization of solar energy is the design and synthesis of photocatalytic materials with narrow band gap energies, high electron–hole separation efficiency, and desired surface structure for the adsorption of reactants [1]. The multifunctional materials ZnO and TiO2 are frequently used together or separately in applications related to thermoelectrics [2], batteries [3], biomaterials [4], supercapacitors [5], sensors [6], and photocatalysis [7,8,9,10,11,12] due to their chemical/photochemical/thermal stability, biocompatibility, desired semiconducting properties, and low cost. When used alone, both photocatalysts have limited photocatalytic activities which, however, can be improved by doping with various metals/nonmetals [7] combining with other semiconductors [12] or with each other [8,9,10, 13,14,15] to form composites. ZnO/TiO2 composite powders [9, 10, 12] and thin films [14, 16] were found to exhibit greater photocatalytic activities than their constituents.
TiO2 and ZnO are limited in their ability to absorb light by their respective band gap energies of 3.2 and 3.4 eV [15]. Combining ZnO and TiO2 to create a composite structure can lower the band gap energy and boost light absorption capacity [14]. The electron excitation from the valence band to the conduction band is made easier by the decrease in band gap energy, which could ultimately lead to an increase in photocatalytic activity. Therefore, the enhancement of the photocatalytic activity can be related to the decreased band gap energy and the efficient separation of electron–hole pairs [9, 14] in the heterostructured ZnO/TiO2 composite photocatalyst. Homogeneous phase distribution and providing suitable boundaries for the efficient contact of these two different phases can be achieved by the simultaneous synthesis of ZnO and TiO2 through new approaches including in situ phase transformation [17,18,19,20,21].
ZIF-8/TiO2 composites can be used as ZnO/TiO2 precursors since they were reported to have greater photocatalytic activities compared to their constituent components [22,23,24,25,26,27,28,29]. The conversion of ZIF-8 to ZnO occurs during a heat treatment step that is typically applied above 400 °C in an air [20, 21] or nitrogen atmosphere [19]. Additionally, by adjusting the annealing temperature, the composite thin film's physicochemical characteristics, including crystallinity, roughness [30] thickness, and band gap energy may be modified [31]. The organic component from ZIF-8 or the titanium precursor present in the structure might operate as a template, which could be removed by annealing the material at various temperatures, increasing the porosity and, consequently, the catalytic activity [32]. ZIF-8 is a high surface area metal organic framework (MOF) which is composed of imidazole rings and zinc atoms [27]. Carbon and nitrogen atoms originating from the imidazole rings may remain in the structure after pyrolysis/combustion [17] resulting in band gap energy reduction due to nonmetal doping and porosity increase due to the organic groups leaving the structure. Thin film technology offers a number of benefits for photocatalysis-based water treatment systems, including low material costs, ease of operation, long-term stability, and a simpler method of photocatalyst recovery [33]. There are limited number of studies on the photocatalytic applications of ZnO-containing composite films derived from ZIF-8 nanoparticles. Wee et al. [34] prepared a composite film by the local transformation of ZIF-8 to ZnO nanorods for their use in photocatalytic applications. The transformation was conducted through a series of chemical reactions including dehydration and condensation induced by Ag+ ions. Nanoparticles were coated on a carbon paper in the study, only nanoparticles were used to assess the photocatalytic activity. The number of publications involving the opposite, ZnO–ZIF-8 transformation and photocatalytic applications, is also very limited [35].
In this study, ZnO/TiO2 thin films were derived from ZIF-8/TiO2 thin films and their photocatalytic activities were determined. ZIF-8 and Degussa P25 were used as ZnO and TiO2 precursor and source, respectively, and titanium tetraisopropoxide (TTIP), an organic titanium source, was used as the precursor of main TiO2 matrix and for the proper adhesion of both ZIF-8 and P25 nanoparticles on the glass substrates. As a result, a ZnO/P25/TiO2 nanocomposite thin film was obtained prior to annealing. With an easy and controllable method, several advantages such as band gap energy reduction by nonmetal doping, creation of a porous structure with carbon removal, and efficient charge separation on the oxide–oxide heterostructure have been provided by the transformation of ZIF-8/TiO2 to ZnO/TiO2.
2 Materials and methods
2.1 Chemicals
Degussa P25 (Evonik) and titanium tetraisopropoxide (TTIP, Aldrich 97%) were used as TiO2 source and precursor, respectively. Zinc nitrate hexahydrate (98%, Aldrich) and 2-methylimidazole (Hmim, 99%, Aldrich) were used as ZIF-8 precursors. Ethanol (absolute) and methanol (absolute) obtained from Aldrich were used in the synthesis of ZIF-8 nanoparticles. Distilled water was used in the photocatalytic dye degradation experiments. In the radical capture studies, di-ammonium oxalate monohydrate (Merck, 99%), p-benzoquinone (Sigma-Aldrich, 98%), and iso-propyl alcohol (ISOLAB, 99.5%) were used. Caffeic acid (98%) was obtained from Sigma-Aldrich.
2.2 Synthesis of ZIF-8 nanoparticles
ZIF-8 nanoparticles were prepared by the rapid addition of 0.07 M, 45 mL methanolic zinc nitrate solution to 0.56 M, 45 mL methanolic Hmim solution under vigorous stirring. The molar ratio of Hmim:Zn2+ was 8:1 and the mixture was stirred at room temperature for 30 min as reported elsewhere [28].
2.3 Synthesis of thin films
Degussa P25 (P25) nanoparticles (0.5 g) were first dispersed in 50 mL ethanol in an ultrasonic bath for 30 min. A portion of P25 suspension (20 mL) was taken and then mixed with 10 mL ZIF-8 suspension taken from previously prepared ZIF-8 suspension. The obtained suspension was diluted by adding 30 mL absolute ethanol. Thus, a 60 mL suspension containing P25 and ZIF-8 nanoparticles was obtained. TTIP (4 mL) was subsequently added for the formation of main TiO2 matrix which also contributed to efficient binding of P25 and ZIF-8 nanoparticles on the glass surface. Theoretically, the weight percentage of ZIF-8 in the final ZIF-8/P25/TiO2 structure was 6.1 wt% if it is assumed that all the zinc in zinc nitrate reacted to form ZIF-8 and TTIP was all converted to TiO2. In the synthesis, Degussa P25 was used as an additional TiO2 source to incorporate rutile phase in the structure due to its narrower band gap energy and efficient cooperation with anatase phase in the photocatalytic reactions. Also, the energy required for the transformation of anatase to rutile phase through TTIP oxidation can be provided at temperatures mostly above 550 °C, and this temperature cannot be used for annealing since it would soften the support borosilicate glass. Therefore, Degussa P25 which already contains rutile phase was added in the recipe. To achieve a uniform and continuous surface coating, a clean surface is crucial. In an ultrasonic bath, the glass substrates (2.5 × 7.5 glass slides) were soaked in detergent water, distilled water, acetone, and ethanol, respectively. Ethanol was then passed over the slides before drying with nitrogen flow. Coating was performed by a dip coater (TDC-10, Teknosem) with dipping-pulling rates of 200–200 mm/min. Coating was repeated several times to increase the thin film thickness (1–10 cycles). A certain time was waited between coating cycles for the solvent removal and the coating suspension was stirred during this time to ensure homogeneity. After completion of the coating cycles, ethanol was then passed over the films to remove unused reactants from ZIF-8 synthesis. As a result, ZIF-8/P25/TiO2 thin films were prepared by these successive steps. These films were dried at 80 °C for 24 h (this film was coded as as-prepared) and then annealed at 200, 300, 400, and 500 °C for 3 h for the transformation of ZIF-8 to ZnO and obtain ZnO/P25/TiO2 thin films. The synthesis procedure is schematically shown in Fig. 1. The name of the thin film was abbreviated as ZnO/TiO2 since P25 is pure TiO2.
2.4 Characterization experiments
Optical characterization of the prepared films was carried out with Agilent Cary60 UV–Vis Spectrophotometer. The transmittance of the films was recorded at the 300–1000 nm wavelength range. The band gap energies of the films were calculated using Tauc curves. The Tauc curves were constructed according to the relations given below:
where hv is the photon energy, β is a constant, Eg is the band gap energy, n is 0.5 for indirect band gap, and α is the absorption coefficient which was calculated using Eq. 2 where T is the transmittance and d is the film thickness. Characterization of the crystalline phase structure was carried out by X-ray diffraction (XRD) analysis with Rigaku MiniFlex 600 for all the films and PANalytical EMPYREAN XRD equipment for the thin film annealed at 500 °C with monochromated high-intensity (λ = 1.54 Å) CuKα radiation. Crystallite size was calculated by using Scherrer’s equation [29]. Surface morphology and film thickness were investigated by FEI Quanta 650 scanning electron microscope (SEM) with an operating voltage of 20 kV. The thickness was calculated by the software program ImageJ 1.50b [36]. Chemical characterization of the films by X-ray photoelectron spectroscopy (XPS) was performed using XPSSPECS EA 300. Carbon C1s peak at 284.8 eV was taken as a reference for the determination of accurate peak positions. Peak fitting was performed by using the Gaussian–Lorentzian function with XPSPEAK41 software program. Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectrum in the 400–4000 cm−1 wavenumber range was obtained with Jasco FT/IR-6800.
2.5 Photocatalysis experiments
Photocatalytic dye degradation experiments were carried out with the photoreactor system schematically shown in Fig. 2. LED lamp which was cooled by a computer processor fan and aluminum heat sink was placed about 5 cm away from the beaker (50 mL) containing 2 ppm methylene blue solution or 10 ppm caffeic acid (CA) solution. The determination of the number of layers and annealing temperature experiments (Sect. 3.2.1) were conducted with a 10 W, 365 nm LED lamp, whereas the comparison (Sect. 3.2.2), reusability (Sect. 3.2.3), and radical capture (Sect. 3.2.4) experiments were conducted with the same setup except the light source was 10 W, 390–395 nm LED lamp. The concentrations of radical scavengers in the radical capture experiments were 0.4 mM for each scavenger.
The solution was stirred continuously during the experiments. Samples were analyzed by UV–Vis spectrophotometer (Agilent, Cary60 UV–Vis Spectrophotometer). Percent dye degradation was calculated by the following equation:
where I is the absorbance of the samples and Io is the absorbance of 2 ppm methylene blue solution at 664 nm wavelength. The 1st order reaction rate constant was calculated according to the following equation:
where C0 is the initial MB concentration, Ct is the MB concentration at time t, and k is the reaction rate constant. The photocatalytic experiments of the thin film annealed at 500 °C were repeated four times.
3 Results and discussion
3.1 Characterization
3.1.1 XRD and FTIR analysis
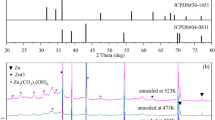
XRD patterns of the thin films in the of 5°–40° 2theta range are given in Fig. 3a, and the XRD pattern of the thin film annealed at 500 °C obtained with PANalytical EMPYREAN is given in Fig. 3b. Reference XRD patterns (selected main peaks listed in the corresponding JCPDS files) are also given in Fig. 3b. The peak at ~25.2° 2theta corresponding to anatase (101), phase of TiO2 was found to be the main peak in the XRD pattern of the thin film annealed at 500 °C. The same peak with a very low intensity was found in the pattern of the thin film annealed at 400 °C which indicated a crystalline TiO2 phase was developed at low levels and/or with low crystallite size. XRD patterns of the as-prepared and 200–300 °C annealed thin films indicated the absence of crystalline phases which may be due to low annealing temperature and low amounts of crystalline P25 and ZIF-8 nanoparticles. ZnO phases and rutile TiO2 phase from P25 or the oxidized TTIP were also not detected in the XRD pattern (Fig. 3b) of the thin film annealed at 500 °C. The reason may be the relatively lower amounts (out of XRD detection limits) of ZnO or P25 in the nanostructure, no rutile formation at this annealing temperature or formation at very low amounts from TTIP oxidation. There are slightly visible peaks detected at ~36.7° to ~38.5° 2θ range which may be attributed to (004)/(112) planes of TiO2 according to the reference TiO2 pattern given in Fig. 3b. The relatively low amounts of the crystalline phases containing these planes and the possibility of peak shifts made it difficult to determine the accurate peak positions and to show the presence of ZnO phases. However, the presence of Zn and N species originating from ZIF-8 in the films was shown by XPS analyses which will be presented in the following sections. The crystallite size of anatase TiO2 in the thin film annealed at 500 °C was calculated to be 27 nm. FTIR analysis was carried out in order to determine whether ZIF-8 was completely transformed into ZnO during annealing process. The FTIR spectrum of the thin film annealed at 500 °C is shown in Fig. 4. The FTIR spectrum contains typical absorption bands of metal oxygen bonds in the 1250–500 cm−1 wavenumber range originating from mostly Ti–O bonds that dominate the nanostructure [18]. The IR bands at around 1600 cm−1 and 3400 cm−1 originate from the –OH bonds of adsorbed water or hydroxyl groups on the surface [37]. The IR bands of C=N stretching (~1580 cm−1), C–N stretching (~1420 cm−1 and ~990 cm−1) [38], imidazole bending/stretching (~1310 cm−1, ~1150 cm−1 and ~1450 cm−1, ~1380 cm−1), and the bands of aromatic sp2 C–H (~760 cm−1 and ~690 cm−1) [39] which may originate from ZIF-8 were not detected in the spectrum. These results indicated that ZIF-8 completely transformed into ZnO during annealing at 500 °C. XPS analysis was further conducted to confirm the removal/transformation of nitrogen-containing bonds originating from ZIF-8. The results showed that pyridinic nitrogen (–NH–) in ZIF-8 was transformed into oxidized nitrogen due to annealing at 500 °C (Fig. 7).
3.1.2 SEM analysis
The photographs and SEM images of the thin films are presented in Figs. 5 and 6, respectively. The photographs (Fig. 5) indicated the formation of homogeneous and semi-transparent thin films. The color of the as-prepared thin film was white since ZIF-8 and P25 TiO2 are white powders and TTIP is transparent; however, the color of the thin films changed from white to brown with varying shades with the increase in the annealing temperature up to 500 °C at which the color turned to white again. This indicated that the burning of organic groups was completed at 500 °C. The thickness of the as-prepared film gradually decreased from 570 ± 78 to 259 ± 28 nm with the increasing annealing temperature as can be seen from Fig. 6. The reason may be the removal of organic phase and densification through crystalline phase formation during annealing. The spherical-like nanoparticles (ZIF-8 and P25) are well dispersed through the TTIP/TiO2 matrix in all thin films. A porous structure was formed due to solvent removal during drying of the as-prepared film. It was observed that the pore structure was preserved when the as-prepared thin film was annealed. The presence of amorphous TTIP matrix can be observed in the SEM images up to 400 °C at which temperature the amount of residuals may be at low levels. The oxidation of amorphous Ti(OH)4 formed by the hydrolysis of TTIP is completed at about 500 °C [40] which is also supported by the photographs and SEM images of the thin films prepared in this study. The SEM image of the surface of the thin film annealed at 500 °C indicated the complete disappearance of organic phase and formation of a porous network structure connected by the nanoparticles with specific shapes. A significant decrease in the thickness was observed due to carbon-based compounds leaving the structure via the complete decomposition of the organic phase mostly originating from the titanium precursor TTIP and the densification of the newly formed TiO2 and ZnO crystalline nanoparticles together. The disappearance of the carbon layer from the network and the presence of nanoparticles of different sizes (anatase and rutile from Degussa P25, anatase from the oxidation of TTIP and ZnO from ZIF-8) may also form an interparticulate network structure. This was also demonstrated with organic [41], carbon [42] templates or by using building blocks of different sizes [43]. The differences in the morphology seen in the surface SEM images of the thin films annealed at low temperatures and 500 °C show the removal of carbonaceous layer revealing the nanoparticles which may be an indication of the formation of interparticulate pores as reported by Cho et al. [42]. The average particle size in the thin film annealed at 500 °C was estimated to be 35 ± 7 nm from a set of nanoparticles with sizes in the 14–54 nm range.
3.1.3 XPS analysis
XPS spectra of the thin films in the C1s, Ti2p, O1s, Zn2p, and N1s regions and the determined peak positions are given in Fig. 7. The deviation of the C–C bond energy, which is the main peak with a binding energy of approximately 283.4 eV for the films, from 284.8 eV was used as a reference to determine the accurate binding energies of all other elements. The spectra in all regions were corrected by adjusting the C–C binding energy to 284.8 eV. The fitted C1s spectra consist of 3 peaks in the 284.8–288.8 eV range corresponding to C–C, C–O, and C–N for the thin films. Carbon atoms in C–C bond (284.8 eV) may originate from impurity carbon and the imidazole ring, C–N bond (285.2 and 285.5 eV) may originate from the imidazole ring, whereas the carbon atoms in C–O bond (288.5 and 288.8 eV) may originate from adsorbed hydroxyls [24, 44]. The ratios of the peak areas of C1s to Ti2p3/2 calculated by using the corresponding atomic sensitivity factors [45] were determined to be 8.8, 3.4, 4.5, 11.3, and 2.8 for the as-prepared, 200, 300, 400, and 500 °C annealed thin films, respectively. This finding indicated the removal of trapped solvent carbon and a portion of the organic content of titanium precursor TTIP with annealing at 200 and 300 °C (among which carbon content slightly increased by the increase in temperature), carbon deposition in the 400 °C annealed thin film which may be due to initiation of the decomposition of TTIP and removal of relatively high amounts of carbon from the structure at 500 °C which was also evidenced from the photographs of the thin films in Fig. 5. Ti2p spectra of the thin films were fitted with similar peaks in the 458–464 eV range. The separation between Ti2p3/2 and Ti2p1/2 is 5.7 eV, which is consistent with the standard binding energies of Ti–O bond in TiO2 [26]. O1s spectrum of the as-prepared thin film was fitted with two peaks, whereas the O1s spectra of the annealed thin films were fitted with three peaks in the 529–536 eV range. The first peaks located at ~529 to 530 eV can be attributed to oxygen in the TiO2 or ZnO (for the annealed films) lattice and the peaks at ~531 to 532 eV can be attributed to oxygen in the –OH bonds, respectively [12]. The third peak located at ~535 eV in the XPS spectra of the annealed thin films may correspond to adsorbed H2O [46]. Zn2p spectra of the thin films were fitted with two peaks originating from Zn2p3/2 and Zn2p1/2, located at ~1022 and ~1045 eV, respectively [18]. N1s spectra of the thin films were fitted with two peaks located in the 398.7–406.8 eV range. The peaks at ~399.5 and ~400.5 eV in the spectra of the as-prepared and annealed thin films at 200–400 °C correspond to pyrrolic nitrogen in –NH– and pyridinic (N bonded to two carbon atoms) nitrogen in the imidazole ring, respectively. The N1s spectrum of the thin film annealed at 500 °C was fitted with two peaks at 399.3 and 406.8 eV corresponding to pyrrolic nitrogen in –NH– bond and oxidized nitrogen, respectively [44]. Although the spectrum of the thin film heat treated at 400 °C appears to be cut at 407.2 eV (which may be due the relatively large shifts in the binding energies of this film possibly originating from high carbon amount on the surface; all N1s spectra containing raw data were recorded in the 391–411 eV range), no signs of peak formation due to oxidized nitrogen (at ~406 to 407 eV) were observed in the N1s spectrum of this film. On the other hand, the N1s spectrum of the thin film annealed at 500 °C, indicated that pyridinic nitrogen transformed to oxidized nitrogen through annealing at 500 °C. This oxidized nitrogen may be trapped among the grains in the nanostructure. But it is more likely that nitrogen may be doped and form N–O–Zn or N–O–Ti bonds in the crystal lattices of ZnO or TiO2 phase boundaries where ZnO and TiO2 nanoparticles are in contact. New energy levels introduced by N doping in TiO2 or ZnO lattices may have decreased the band gap energy of the thin film annealed at 500 °C and contributed to the enhancement in the photocatalytic activity.
3.1.4 UV–Vis spectroscopy analysis
The transmittance curves and Tauc plots generated for the calculation of the band gap energies of the thin films are given in Fig. 8. The transmittance curves shifted to visible light region with increasing annealing temperature. The optical band gap energies of the thin films were determined by plotting the incident photon energy (hv) against (αhv)n where n is equal to 0.5 for indirect band gap. The band gap energies were calculated to be in the 2.04–3.23 eV range. The band gap energies decreased with increasing annealing temperature as shown in Fig. 8b. The narrowest band gap energy (2.04 eV) was obtained with the film annealed at 500 °C. The significant change in the band gap energy with annealing temperature may be attributed to the formation of crystalline ZnO phase and incorporation of nonmetals to ZnO or TiO2 lattice on the boundaries of these phases due to oxidation of carbon/nitrogen-containing ZIF-8 nanoparticles. The increased porosity and the formation of a defective structure caused by the removal of carbon/nitrogen-containing species during crystalline phase evolution may also be involved in the reduction of the band gap energy [17]. Band gap energy is an important parameter in photocatalysis that shows how much the photocatalyst absorbs light. The absorption of sunlight in a wide range of UV, visible, and IR regions and efficient utilization has significant effects on the activity of the photocatalyst [1]. By enabling more light to be utilized, having a narrow band gap energy increases the photocatalytic activity, not always, but often. The results showed that the light absorption capacity of the thin film annealed at 500 °C is higher than the other thin films. This indicates that the photocatalytic activity of this thin film is likely to be more (as shown in the performance results) than the other thin films.
3.2 Photocatalytic properties
3.2.1 Determination of the optimum number of layers and annealing temperature
The effects of number of layers and annealing temperature were investigated in the photocatalytic degradation of methylene blue dye (MB) under 365 nm LED light irradiation. Thin films with different number of layers were first prepared in order to determine the maximum number of layers that provides high photocatalytic activity without disturbing the continuity and homogeneity of the thin film structure. The photocatalytic MB degradation curves of the thin films with different number of layers are shown in Fig. 9a. The annealing temperature was set to 400 °C for this group before the evaluation of the effects of annealing temperature on the properties and photocatalytic activity. The activity gradually increased when the number of layers was increased. The activities of the thin films containing 5 and 7 layers were found to be similar whereas the activity decreased when the number of layers was increased to 10. It was observed that there were cracks and ruptures on this film most probably due to high film thickness. Thus, 5 was selected as the optimum number of layers to further evaluate the effect of annealing temperature.
The effects of annealing temperature on the photocatalytic properties of the thin films are shown in Fig. 9b–d and Table 1. The photocatalytic MB degradation curves (Fig. 9b) showed that the activity gradually increased with temperature at lower annealing temperatures (200–400 °C), while it significantly increased at 500 °C. The photocatalytic MB degradation efficiencies were calculated to be 3%, 15%, 33%, and 72% (± 2%) for the films annealed at 200 °C, 300 °C, 400 °C, and 500 °C, respectively (Table 1). The 1st order reaction rate constant increased by 3 folds when the annealing temperature was increased to 500 °C from 400 °C as shown in Fig. 9d. The fold-difference was larger when the rate constant obtained at 500 °C was compared with those of lower annealing temperatures. The significant increase in the 1st order reaction rate constant indicates that the thin film annealed at higher temperature photocatalytically degrades MB molecules faster than the other thin films. In terms of application, this will create a great advantage and shorten the duration of photocatalysis step in water treatment process which eventually may reduce process costs. The photographs (Fig. 5) and SEM images (Fig. 6) of the thin films annealed at 200–400 °C showed that there were significant carbonaceous layers in the nanostructure most probably due to unburned organic species originating from ZIF-8 or TTIP. Carbonaceous layer acting as template and forming connected and free pores after annealing at high temperature may be preserved at lower annealing temperatures as seen in the SEM images. This presence may occlude pores and restrict the transport of MB molecules and cause the reaction take place only on the pore mouth [32]. It was found that these layers were removed at 500 °C and distinctive/exposed nanoparticles appeared which may be an indication of the formation of a more porous structure at this temperature. The obtained high photocatalytic activity at 500 °C may be attributed to the increased porosity which may have enhanced the transport/adsorption of MB molecules, utilization of more photons due to narrower band gap energy, and decelerated electron–hole recombination which may increase the concentration of photogenerated radical species at the junctions of ZnO and TiO2 phases [17]. The XPS analysis also indicated an N-doped crystalline structure which may have led to the formation of defect zones facilitating the electron–hole separation which enhances charge carrier transport [8].
3.2.2 Photocatalytic activity comparison
The photocatalytic activity of the thin film annealed at 500 °C was compared in two different aspects. The activity was first compared to the as-prepared ZnO/TiO2 thin film which was not annealed and neat TiO2 which does not contain P25 and ZnO nanoparticles but annealed at 500 °C. The second aspect is the comparison of its activity in the degradation of a colorless pollutant (caffeic acid). The photocatalytic activities of the as-prepared and annealed ZnO/TiO2 thin films along with neat TiO2 thin film annealed at 500 °C are demonstrated in Fig. 10a. The results indicated that the photocatalytic activity of ZIF-8/TiO2 thin film was enhanced by the transformation of ZIF-8 to ZnO via annealing at 500 °C. The reasons for the enhanced degradation may be the increased crystallinity, anatase crystallites are known to be active in the photocatalytic degradation of MB molecules, formation of a porous structure by the decomposition of organic groups in TTIP and ZIF-8, and nonmetal doping due to N atoms originating from the imidazole rings of ZIF-8. It was also observed that the activity increased when P25 and ZnO nanoparticles were incorporated in the structure of TiO2. The possible reason is considered to be the formation of heterojunctions with beneficial properties as discussed in the previous section.
Caffeic acid is classified as a Group 2B carcinogen and can be found as a colorless pollutant in various aquatic effluents [47]. Photocatalytic degradation of colorless pollutants is also important in terms of photocatalytic performance determination. The photocatalytic degradation of caffeic acid with the thin film annealed at 500 °C is demonstrated in Fig. 10b. It was observed that caffeic acid was self-degraded by UV light. However, the degradation accelerated in the presence of the prepared thin film. These findings indicated that this thin film is also active in the photocatalytic degradation of caffeic acid (~60% efficiency) in addition to being active in photocatalytic MB degradation (~70% efficiency).
3.2.3 Reusability and stability experiments
In order to determine the reusability and stability of the thin film annealed at 500 °C, photocatalytic activity determination studies were conducted for four cycles, and XRD and SEM analyses after four cycles were performed. The results of these analyses are given in Fig. 11. The photocatalytic activity did not change significantly after four cycles as seen in Fig. 11a. It was determined that there was a slight decrease in the activity only at the end of the first cycle, but this decrease was not observed after the other cycles. This decrease may be due to the remaining of unreacted MB molecules on the surface after the first cycle (due to ~70% degradation) and initiation of the next cycle with these adsorbed MB molecules. XRD patterns (Fig. 11b) and SEM images (Fig. 11c) of the fresh and used thin film showed that the crystal structure and morphology did not change significantly after four cycles and the prepared thin film maintained its stability.
3.2.4 Radical capture experiments
The photocatalytic MB degradation mechanism was elucidated by determining which of the photogenerated species on the thin film surface actively participate in photocatalysis. Superoxide (.O2-), hydroxyl (.OH), and hole (h+) radicals were scavenged by the addition of p-benzoquinone (BQ), iso-propyl alcohol (IPA), and di-ammonium oxalate monohydrate (AO), respectively, to the MB solution. The addition of superoxide scavenger significantly reduced the photocatalytic degradation rate of MB, while the addition of hole scavenger and hydroxyl scavenger reduced the activity to a relatively lower extent (Fig. 12a and b). When a hydroxyl scavenger was added, the photocatalytic degradation rate decreased more than when a hole scavenger was added, indicating that hydroxyl radicals also participate in the photocatalytic reaction but are less active than superoxide radicals. These findings showed that superoxide radicals are the predominant active species generated by the photogenerated electrons captured by molecular oxygen [48] in the MB solution. The proposed mechanism for the photocatalytic degradation of MB is schematically shown in Fig. 13.
4 Conclusions
ZnO/TiO2 thin films were successfully prepared by annealing ZIF-8/TiO2 thin films at different temperatures. The annealing temperature significantly affected the surface morphology, thickness, band gap energy, and hence the photocatalytic activity of ZnO/TiO2 thin films. The carbonaceous layer formed due to unburned organic species was removed at 500 °C revealing a porous structure. The band gap energy significantly decreased at this temperature which is most probably due to the formation of an N-doped crystalline structure. The highest MB degradation rate (72%) was obtained with the film annealed at 500 °C which has three times higher first-order reaction rate constant compared to the film annealed at 400 °C. The obtained high photocatalytic activity at 500 °C annealing temperature was attributed to the narrow band gap energy, high charge carrier transport due to the decrease in the electron–hole recombination rate by N-doped defective zones and heterojunctions, and high porosity formed due to the removal of carbonaceous layer and possible variable phase densification during crystalline ZnO and TiO2 formation. The results indicated that ZnO/TiO2 thin film prepared by the annealing of ZIF-8/TiO2 thin film shows activity also in the photocatalytic degradation of caffeic acid which is a colorless pollutant. This study has shown that the aforementioned properties along with the photocatalytic activity of ZnO/TiO2 thin films can significantly be modified by the applied annealing temperature of ZIF-8/TiO2 thin films.
Data availability
Data will be made available on request from the authors.
References
G. Hu, J. Yang, X. Duan, R. Farnood, C. Yang, J. Yang, W. Liu, Q. Liu, Chem. Eng. J. 417, 129209 (2021)
C. Soumya, P.P. Pradyumnan, Mater. Today Commun. 35, 106197 (2023)
X. Zhao, Z. Zhang, H. Zhang, X. Xu, X. Fan, S. Wang, Int. J. Electrochem. Sci. 17, 22057 (2022)
M.Ç. Yurtsever, G. Güldağ, J. Mech. Behav. Biomed. Mater. 146, 106088 (2023)
A. Ehsani, M. Bigdeloo, H. Alamgholiloo, E. Asgari, A. Sheikhmohammadi, S. Nazari, B. Hashemzadeh, N. Ghasemian, J. Energy Storage 50, 104633 (2022)
Y. Liu, C. Quan, Int. J. Electrochem. Sci. 18, 100308 (2023)
K. Choudhary, R. Saini, L.P. Purohit, Opt. Mater. (Amst). 143, 114167 (2023)
K. Gowthaman, P. Gowthaman, M. Venkatachalam, M. Saroja, M. Kutraleeswaran, S. Dhinesh, Inorg. Chem. Commun. 146, 110197 (2022)
J. You, L. Zhang, L. He, B. Zhang, Opt. Mater. (Amst). 131, 112737 (2022)
S.Z. Heris, M. Etemadi, S.B. Mousavi, M. Mohammadpourfard, B. Ramavandi, J. Photochem. Photobiol. A: Chem. 443, 114893 (2023)
F. Zheng, J.M. Queirós, P.M. Martins, R.F. de Luis, A. Fidalgo-Marijuan, J.L. Vilas-Vilela, S. Lanceros-Méndez, J. Reguera, Colloids Surf. A: Physicochem. 671, 1 (2023)
J. Zhang, M. Kuang, Y. Cao, Z. Ji, Solid State Sci. 129, 106913 (2022)
A.S. Rad, A. Afshar, M. Azadeh, Opt. Mater. (Amst). 136, 113501 (2023)
E. Widyastuti, C.T. Chiu, J.L. Hsu, Y. Chieh Lee, Arab. J. Chem. 16, 105010 (2023)
Y. Yadawa, S. Singh, A. Ranjan, Mater. Sci. Eng. B 288, 116164 (2023)
D. Upadhaya, P. Kumar, D. DharPurkayastha, J. Mater. Sci. Mater. Electron. 30, 10399 (2019)
C. Cheng, J. Wang, Z. Zhao, C. Chen, S. Cui, Y. Wang, L. Pan, Y. Ni, C. Lu, J. Alloys Compd. 896, 163064 (2022)
Q. Jiang, Z. Han, Y. Qian, Y. Yuan, Y. Ren, M. Wang, Z. Cheng, J. Water Process Eng. 47, 102768 (2022)
W. Li, Y. Chen, W. Han, S. Liang, Y. Jiao, G. Tian, Sep. Purif. Technol. 309, 122970 (2023)
E. Shamsaei, F.B. de Souza, K. Sagoe-Crentsil, W. Duan, Microporous Mesoporous Mater. 332, 111702 (2022)
X. Yang, J. Pan, B. Xing, Z. Xie, Y. Fu, K. Cheng, Arab. J. Chem. 16, 104789 (2023)
R. Chandra, S. Mukhopadhyay, M. Nath, Mater. Lett. 164, 571 (2016)
E. Pipelzadeh, V. Rudolph, G. Hanson, C. Noble, L. Wang, Appl. Catal. B Environ. 218, 672 (2017)
R. Li, W. Li, C. Jin, Q. He, Y. Wang, J. Alloys Compd. 825, 154008 (2020)
X. Qi, F. Shang, T. Wang, Y. Ma, Y. Yan, Y. Yan, CrystEngComm 22, 4250 (2020)
W.L. Zhong, C. Li, X.M. Liu, X.K. Bai, G.S. Zhang, C.X. Lei, Microporous Mesoporous Mater. 306, 110401 (2020)
S. Angela, V.B. Lunardi, K. Kusuma, F.E. Soetaredjo, J.N. Putro, S.P. Santoso, A.E. Angkawijaya, J. Lie, C. Gunarto, A. Kurniawan, S. Ismadji, Environ. Nanotechnol. Monit. Manag. 16, 100598 (2021)
H.A. Yurtsever, A.E. Çetin, Colloids Surf. A Physicochem. 625, 126980 (2021)
Z. Liu, Y. Zhong, Z. Hu, W. Zhang, X. Zhang, X. Ji, X. Wang, J. Mol. Liq. 380, 121729 (2023)
M.C. Wang, H.J. Lin, C.H. Wang, H.C. Wu, Ceram. Int. 38, 195 (2012)
C.P. Lin, H. Chen, A. Nakaruk, P. Koshy, C.C. Sorrell, Energy Proc. 34, 627 (2013)
M. Pirouzmand, M. Asadi, A. Mohammadi, Greenh. Gases Sci. Technol. 8, 462 (2018)
V. Deepthi, A. Sebastian, B. Vidhya, J. Mater. Sci. Mater. Electron. 33, 24111 (2022)
L.H. Wee, N. Janssens, S.P. Sree, C. Wiktor, E. Gobechiya, R.A. Fischer, C.E.A. Kirschhock, J.A. Martens, Nanoscale 6, 2056 (2014)
M. Kräuter, K. Unger, R. Resel, A.M. Coclite, Coatings 13, 1 (2023)
C.A. Schneider, W.S. Rasband, K.W. Eliceiri, Nat. Methods 9, 671 (2012)
S. Sunaryono, S.N. Halizah, S. Zulaikah, H. Susanto, N. Mufti, A. Taufiq, AIP Conf. Proc. 030013, 2 (2023)
M. Zhang, Q. Shang, Y. Wan, Q. Cheng, G. Liao, Z. Pan, Appl. Catal. B Environ. 241, 149 (2019)
R. Fazaeli, H. Aliyan, J. Iran. Chem. Soc. 16, 1 (2019)
P. Paunović, A. Grozdanov, A. Češnovar, P. Makreski, G. Gentile, B. Ranguelov, E. Fidančevska, J. Eng. Mater. Technol. Trans. ASME 137, 1 (2015)
J.Y. Ahn, X. Luo, S.H. Kim, Microporous Mesoporous Mater. 266, 214 (2018)
T.Y. Cho, C.W. Han, Y. Jun, S.G. Yoon, Sci. Rep. 3, 1 (2013)
S. Palvai, D. Kpeglo, G. Newham, S.A. Peyman, S.D. Evans, Z.Y. Ong, ACS Appl. Mater. Interfaces 16, 5568 (2024)
E.D. Miensah, J. Chen, A. Gu, P. Wang, Y. Liu, C. Gong, P. Mao, K. Chen, Y. Jiao, Z. Zhang, Y. Yang, J. Solid State Chem. 299, 1 (2021)
C.D. Wagner, J. Electron Spectros. Relat. Phenomena 32, 99 (1983)
A. Wiatrowski, M. Mazur, A. Obstarczyk, D. Wojcieszak, D. Kaczmarek, J. Morgiel, D. Gibson, Coatings 8, 1 (2018)
F. Puga, J.A. Navío, M.A. Paulete-Romero, J.M. Córdoba, M.C. Hidalgo, Mater. Sci. Eng. B 276, 1 (2022)
X. Yu, Z. Song, X. Dong, H. Li, H. Liu, B. Zhao, J. Mater. Sci. Mater. Electron. 33, 13271 (2022)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by Adana Alparslan Türkeş Science and Technology University Scientific Research Coordination Unit, Türkiye. Project Number: 19103015. Onur İloğlu was supported by TÜBİTAK 2210-C National MSc/MA Scholarship Program in the Priority Fields in Science and Technology, Türkiye.
Author information
Authors and Affiliations
Contributions
Hüsnü Arda Yurtsever contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Onur İloğlu. The first draft of the manuscript was written by Onur İloğlu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
İloğlu, O., Yurtsever, H.A. The effects of annealing temperature on the thickness, morphology, band gap energy, and photocatalytic performance of ZIF-8-derived ZnO/TiO2 thin films. J Mater Sci: Mater Electron 35, 1211 (2024). https://doi.org/10.1007/s10854-024-12932-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12932-7