Abstract
Atomic layer deposition (ALD), a high-conformality thin-film deposition technique, offers the opportunity to immobilize photocatalytic materials on high surface area substrates. Textile substrates are inexpensive, easily accessible materials with a fibrous nature, making them high surface area scaffolds for photocatalytic applications. This study applied ZnO thin-film coatings to fabric structures with different numbers of ALD cycles. The effect of coating thickness on the surface and electronic properties of the films and their photocatalytic properties were investigated. SEM, XRD, PL, and UV–Vis were used to examine the surface morphology, crystal structure, defects, and optical properties of the ZnO thin films. As the film thickness increased, the crystal sizes and the number of defects in the structure increased. Contact angle and Hall Effect measurements revealed that these structural defects are present on the surface of the films. Optimum wettability, mobility, and photocatalytic efficiency values were observed in the 15-nm coated samples, resulting in the highest photocatalytic activity and a turning point.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photocatalysis is an efficient and promising method for various applications, including wastewater treatment, hydrogen production, air purification, and antibacterial activity. This method relies on the interaction between light energy and a photocatalyst material to drive chemical reactions. Photocatalysis has gained significant interest due to its cost-effectiveness, mild operating conditions, and high efficiency compared to other methods [1]. In recent years, research efforts have focused on developing new photocatalyst materials with enhanced properties, such as high photocatalytic activity, stability, and large surface areas [2]. Metal oxides and semiconductors like titanium dioxide, zinc oxide, silver, copper, copper oxide, cerium, iron oxide, and gold have been extensively studied as potential photocatalysts for water treatment [3,4,5,6,7,8,9,10].
Utilizing solar radiation for water purification faces a significant challenge due to the limited utilization of the visible part of the light spectrum. Researchers have explored hybrid structures by doping semiconductor photocatalyst materials with other elements or materials to address this limitation, extending their absorbance into the visible spectrum [11,12,13]. Another drawback of using photocatalyst materials in powder form is their removal from water during treatment, necessitating additional steps to improve efficiency when using UV rays, mainly due to the larger surface area of powdered materials [14,15,16,17]. To overcome these issues, immobilizing photocatalytic materials onto supporting substrates has been proposed as a potential solution [4, 18]. This approach aims to enhance the efficiency of the photocatalytic process and prevent the loss of materials during water treatment. Recent studies have focused on immobilizing photocatalyst nanoparticles on supporting substrates, including carbon nanotubes, graphene oxides, glass, ceramic materials, polymers, zeolites, and textile materials [19,20,21,22,23]. The demand for materials with excellent stability and high surface area has driven exploration in this direction. Various immobilization techniques have been used, such as the dip coating method [24], electrophoretic method [25], sol–gel [2], solvothermal [26], and spray pyrolysis [27]. Additionally, thermal evaporation film deposition [28], chemical vapor deposition (CVD) [29], and atomic layer deposition (ALD) [30] are also employed techniques for immobilizing photocatalytic materials. These advances hold promising potential for enhancing the efficiency and effectiveness of photocatalysis in water purification applications. Textile substrates promise significant potential as candidate substrates since they are inexpensive and have relatively high surface area [31,32,33]. To manipulate this high surface area at the highest potential, the conformality of ALD technology poses important advantages over other techniques.
The atomic layer deposition (ALD) method is a chemical thin-film technique based on precursor reactions with the substrate’s surface in the vapor phase. This method enables the formation of conformal and uniform thickness films, even on substrates with high surface area, due to the surface reactions involved [34,35,36]. Consequently, applying functional material coatings on the fibrous structures of textile materials, which typically have a relatively high surface area, holds great potential, especially in applications where surface area plays a crucial role, such as catalysis [37,38,39]. Although some studies in this direction exist in the literature, further investigations are needed to explore these films’ structural properties and different compositions.
This study investigated the crystal structures and defects deposited on glass fabrics of ZnO thin films via the ALD method with different thicknesses (5, 10, 15, 25, 35, and 60 nm). Inexpensive textile substrates provided relatively high surface area, while ALD provided conformal coatings on the substrate. It is known in the literature that the thickness of the ALD films affects the photocatalytic performance on planar substrates [40, 41]. However, the film thickness effect is expected to be different on fabric substrates due to the complex morphology of the fabrics. Thus, we systematically investigated and discussed the relationship between photocatalytic activity and structural parameters by varying the number of ALD cycles on fabric substrates. The films were characterized using SEM, XRD, UV–Vis, and PL analyses. We also analyzed the electronic properties of the films through Hall Effect measurements. Additionally, we examined the wettability behavior of the films by measuring the contact angle. Finally, we assessed the films’ photocatalytic activities using two different pollutant models. Our analyses gave insights into how these properties influenced the films’ surface and electronic properties on fabric substrates, resulting in different performances.
2 Experimental
Glass fiber fabrics (Hexcel®, plain weave, 200 gsm) were obtained from a local vendor and used as received. In addition to the fabric samples, 1 × 1 cm pieces of Si wafer (Nanografi), 2.5 × 2.5 cm pieces of fused quartz slide (Technical Glass Products), and 2.5 × 2.5 cm pieces of glass slide were also coated for various characterizations. Atomic layer deposition (ALD) of the ZnO films was accomplished by utilizing diethylzinc (DEZ) (Sigma-Aldrich) and ultrahigh purity H2O (Sartorius™ Arium™ Ultra High Purity Water System) as Zn and O precursors, respectively. Fabrics were cut into 10 × 10 cm to place into the reactor with the Si wafer, quartz, and glass slides. ALD was conducted in a custom designed and made hot wall flow tube reactor, the details of which were given elsewhere [42]. During the deposition of the ZnO ALD films, DEZ was dosed for 0.5 s, and H2O was dosed for 0.2 s, and each dose was followed by a 60 s purge in each cycle at 150 °C and 1 Torr. The thicknesses of the obtained films are given in Table 1.
FESEM analysis of the fabric samples was conducted at Bilkent University UNAM, using a FEI Quanta 200F microscope at 20–30 kV column voltage. A Bruker D8 Advance system was used for the XRD analysis of the fabrics, using a Cu Kα (λ = 1.54 Å) source. Thicknesses of the ALD films on Si wafers were measured using the Filmetrics F20-UV instrument. UV–Vis spectra of the films deposited onto quartz slides were obtained using a Shimadzu UV3800 spectrophotometer. The absorption spectra of samples were obtained using a diffuse reflectance attachment, while an uncoated glass substrate was used as a reference for the measurements. The films’ Photoluminescence (PL) spectra were taken on the coated fabrics and Si wafers using an ANDOR SR500i-BL PL spectrophotometer. Spectra Physics Nd:YLF brand laser with a wavelength of 349 nm, a pulse width of 5 ns, and a pulse energy of 1.3 mJ was used as the light source. Mobility and charge carrier concentrations of the films were measured at room temperature using the Ecopia HMS 3000 Hall Effect Measurement system. Films were deposited onto fused quartz slides to avoid the substrate effect. A 1 × 1 cm ALD-coated quartz was coated with Ag paste at the corners for cross measurements [43].
Methylene blue (MB) (CAS # 122965-43-9, ISOLAB Chemicals, Germany) and Methyl Orange (MO) (CAS# 547-58-0, ISOLAB Chemicals, Germany) were used to evaluate the photocatalytic activities of ZnO films. Efficacy was evaluated by the degradation of the dyes starting with a concentration of 5 × 10–5 M at neutral pH (~ 7) under a solar simulator (ScienceTech SF-300B) illumination. The solution was left in the dark medium for 30 min to reach absorption–desorption equilibrium prior to light exposure. Solution samples of 2 ml were taken at certain time intervals, and the maximum absorption values were recorded using a HACH DR2800 spectrophotometer. After each measurement, the aliquots were reincorporated into the reaction vessel. MB solution was gently stirred at approximately 300 rpm during the photocatalytic activity tests to ensure the dye’s even dispersion throughout the solution and thermal uniformity. To avoid excessive water evaporation from the MB solution, the reactor vessel was placed into a secondary container with iced water at the bottom of the reactor. The details of the photocatalytic activity tests can be found in our previous work [38].
3 Results and discussion
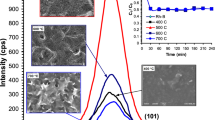
SEM images of uncoated, 5-, 10-, 15-, 25-, 35-, and 60-nm ALD ZnO-coated fibers are given in Fig. 1. The surface of the pristine fiber in Fig. 1a shows a very smooth surface, as expected from the glass fibers. After 5 nm of ZnO ALD, new features form at the fiber surface, as seen in Fig. 1b. As the films’ thickness increases, the films’ granular structure becomes more noticeable in Fig. 1c–g. The 25-, 35-, and 60-nm films in Fig. 1e–g show the columnar growth of the ZnO crystals discussed in the literature [44]. Figure 1h gives low-magnification images of 60-nm coated fibers. Even with the thickest ALD film, all the fibers maintain their independent standing, which is the case for the rest of the samples. This situation indicates that the films are deposited only onto the fiber surfaces as expected from ALD; therefore, the high surface area of the fiber matrix is maintained for photocatalytic activity.
The change in the crystal structure of the ZnO thin films was observed by XRD analysis, and the resulting diffraction patterns are presented in Fig. 2. The fiberglass substrates are made of amorphous glass material; therefore, no crystal peaks were observed. Upon coating the fibers with 5 nm of ALD film, the formation of crystals can be observed with features such as the weak peak at 34.3° associated with the wurtzite structure of the ZnO. Other features of wurtzite are also starting to form, but they are unclear yet. We should mention that the XRD analysis was conducted on the ALD-coated fabric substrates using the Bragg–Brentano arrangement. Since the ALD films are deposited onto the fibers with high surface area and random orientation, the XRD peaks of such thin films can be observed with this arrangement. We think the formation of peaks at very few cycles indicates that the ALD film formation is conformal as expected. After the deposition of 10-nm ZnO onto fabrics, peaks of wurtzite structure are more pronounced in the spectrum, but the peak intensities are still low, and the spectrum has noise. After 15-nm ZnO ALD, the crystal structure began to be observed more clearly, and the intensity of the peaks became more and more evident as the thicknesses increased from 25 to 60 nm. The peaks observed in the XRD analysis were in line with the studies in the literature. The peaks were observed at 2θ angles of 31.98°, 34.61°, 36.50°, 47.80°, 56.86°, 63.05°, and 68.32° hexagonal wurtzite crystal structure of ZnO (100), (002), (101), (102), (110), (103), (112) planes [38, 40, 41]. The characteristic peak positions of the hexagonal crystal wurtzite structure of ZnO determined by JCPDS Card No 00-036-1451 are shown in the graph. As the number of cycles increased, the thickness of the films increased, resulting in the crystal grain growth, as suggested, in crystal peaks.
To further evaluate the crystal structure and defects of the samples, the dislocation density, microstrain, and average crystallite size of ZnO ALD thin films were calculated via Scherrer’s equation using the XRD data and Scherrer’s constant K was taken 0.9 in our study following the literature [38]. According to the results in Table 2, as the film thickness increases, the crystallite size (D) increases, which is plausible since the columnar growth of the wurtzite structure is expected during ALD [41, 45]. According to Scherrer’s equation, an increase in crystallite size decreases dislocation density (δ). Dislocation density and microstrain (ε) are indicators of the dislocation network in the films that are found to be reduced with increasing crystallinity in the ALD films [44]. It is evident that the thicker the ALD films, the larger the crystals, resulting in lower linear defects and strain within the film materials.
The absorption spectra of ZnO ALD thin film were analyzed with a UV–Vis spectrophotometer at room temperature. The graphic in Fig. 3a shows the UV–Vis spectra of the ZnO films deposited with various cycles on the glass substrates. In general, ZnO samples indicate firm absorption peaks between 300 and 390 nm, which is consistent with the studies in the literature [41, 46]. The pristine sample does not show any significant absorption in this range. An absorption behavior starts to be observed for the 5-nm sample with a narrow band and low intensity. Once the thickness of ALD films is increased to 10 nm, absorption becomes more pronounced. As the thicknesses of the ALD films increase, the absorption intensity increases without any significant change in the absorption band, as expected from Lambert–Beer law [41, 46]. The band gap energies of the ZnO films deposited on the glass substrates at various numbers of cycles were calculated by the Tauc and David–Mott method, and the results are given in the graph as shown in Fig. 3b. Samples showed only a slight variation in the bang gap energies in the range of 3.20 and 3.26 eV, indicating the band gap of the films are similar with an average of 3.2 eV. These values are also in agreement with the literature [38, 44].
PL analysis at room temperature was performed on ZnO thin films deposited on Si wafers to observe how the increase in the number of ALD cycles affects the emission behavior of the films. Thus, PL emission behavior can be associated with defects in the structure. The results are given as two plots for thinner and thicker samples in Fig. 4a, b, respectively. Generally, two emission signals were observed between 380–400 nm and 550–750 nm. The emission region we follow between 320 and 420 nm is attributed to the near band edge (NBE) emission with an energy conversion corresponding to the band gap energy of ZnO. This emission occurs due to the rapid recombination of free excitons from ZnO, as explained in the literature [47, 48]. It is clear that this emission emerges as the thickness of the films was increased to 10 nm as shown in Fig. 4a. This may be due to the ultra-thin film formed for the 5-nm sample not comprising enough structure to show this emission. The broad emission region between 500 and 750 nm is defined as the deep-level (DL) emission, which is associated with the defects in the ZnO structure, such as Zn interstitials (Zni), Zn vacancy (Vzn), O interstitials (Oi), and O vacancy (VO) [49, 50]. In this band of the PL spectra, converting photoexcited electrons from the conduction band to the Vo between ~ 500 and 560 nm results in green emission with lower energy. At ~ 600 nm, however, conversion from the Izn to the VO usually results in orange emission. The red emission at ~ 700–750 nm is due to the conversion from the conduction band to the singly ionized oxygen vacancy as defined by the literature [51, 52]. When we examine the behavior of our samples, the sample deposited with 10 nm of ZnO film shows a low-intensity peak between 500 and 560 nm, while it does not show any peaks between 700 and 750 nm. From 15 to 60 nm, the peak intensity between 500 and 560 nm increases as the number of cycles increases with a broad band in this range. Thus, increasing the thickness of the films results in increasing oxygen vacancy defects in the structure of the films. The emissions between 700 and 750 nm start to be more evident as the thickness is above 25 nm, which results in singly ionized O vacancy in the structures.
The ratio between the DL emission peak intensities of the samples and the NBE emission peak intensities was calculated to examine the concentration of the VO in the structures [47, 53]. The graph in Fig. 5 shows the IDL/INBE ratio as a function of the ALD film thickness. A decrease in this ratio means a reduction in the Vo concentration in the structure. The sample with 5-nm film was not included in the calculation as we could not observe the peaks to be used for calculation. For the rest of the samples, the ratios of IDL/INBE vary between 0.48 and 1.19. The 10-nm sample has a relatively higher ratio, which decreases as the thickness of the samples increases up to 25 nm. While the IDL/INBE ratio gave the minimum value for the 25-nm sample, it rose again at 35- and 60-nm films and reached its maximum value at 60 nm. This graph shows that the Vo concentration in the structure decreases up to 25 nm and then it increases as the thickness increases. Wang et al. in their study in 2020, produced ZnO thin films using DEZ + H2O2 and observed that this rate decreased as the number of cycles increased [47]. We had the film using H2O as the oxygen source in our research. We observed that this ratio increased after a specific number of cycles as the number of cycles increased. This shows how different parameters affect the crystal structures of thin films and the formation of defects in these structures. In this context, the study of J. Yang et al. used H2O and H2O2 as oxygen sources in the production of ZnO thin films and examined the structures of the thin films, confirming the case [54].
Hall Effect measurements at room temperature were made to evaluate the electrical properties of ZnO films. In Fig. 6, the variation of the mobility and the charge carrier concentration values of the samples were given as a function of the film thickness. While the mobility values of 5 and 10-nm samples were recorded as 11.7 and 11.8 cm2 V−1s−1, respectively, the graph peaked at 15 nm and reached the maximum mobility value of 75.1 cm2 V−1s−1. Then, a significant decrease was recorded with a value of 10.6 cm2 V−1s−1 for 25-nm film and slowly decreased as the thickness increased to 60 nm. The charge carrier concentration values showed a slight decrease from 5 to 15-nm films as opposed to the mobility, and the minimum carrier concentration value is recorded with a 15-nm sample. The mobility increased significantly with the 25-nm sample and reached the value of 3.8 × 1017 cm−3 for 35-nm film and remained similar for 60-nm film. The relationship between carrier concentration and mobility can be explained by the decrease in carrier mobility due to the scattering effect between carriers when the charge concentration is increased [53, 55]. A VO presence in the ZnO structure can provide two electrons, so ZnO is generally an n-type semiconductor due to the presence of VO [56, 57]. Therefore, the presence of VO in the ZnO structure, which is usually prepared without additives, affects the carrier concentration [58]. In the results obtained in this study, samples up to 15 nm thickness exhibited p-type conductivity behavior. This can be explained by the decrease in carrier concentration due to this structure’s decrease in VO density. After 25 nm, n-type conductivity was recorded for the samples parallel to the increase in carrier concentration. Although the minimum VO density was recorded in a 25-nm sample with a significant increase in carrier concentration, it can be explained as oxygen defects in the structure significantly affecting the carrier concentration value of the samples. When the Hall Effect results are compared to the PL analysis, we can say that the concentration of oxygen defects in the structure decreases up to 25 nm and increases slightly between 25 and 60 nm. These results are agreeable with the carrier concentration values in the films. From this point of view, we can discuss an optimum value for the density of oxygen defects in the structure for increasing mobility depending on the oxygen defect concentration and carrier concentration in the structure can be achieved.
Contact angle (CA) measurement results of the samples are given in the graph as shown in Fig. 7. Wettability is one of the essential properties that provides insight into the surfaces of solid materials, especially for heterogeneous catalysis [59]. Wetting metal oxide materials with water relates to surface topography and surface defects [60]. In the graph given in Fig. 8, the contact angle of the initially pristine fabric is recorded as 109°, thus exhibiting hydrophobic behavior. After the deposition of 5-nm ZnO, the CA decreased abruptly to 64. After 10- and 15-nm depositions of ZnO, the CA further reduced to 40° and 39° (i.e., the minima in this study), respectively. As the thickness of the ZnO film increased to 25 nm, the CA increased back to 68°. Then, it kept rising to 83° after depositing 35 nm of ZnO. Upon coating samples with 60 nm, the CA increased significantly to a higher value, reaching 136°, so the sample became hydrophobic again. Based on these results, it can be said that the glass fabric, which exhibits hydrophobic behavior, becomes hydrophilic with a few cycles of ZnO thin-film deposition and becomes hydrophobic again with the increased number of cycles. Hu et al., in their study in 2013, investigated the effect of the presence and density of oxygen vacancy defects (VO) on the wettability of ZnO films. It is noted that the contact angle of ZnO films increased due to oxygen vacancy defects and increasing density. In our study, when the VO concentration data we obtained from the samples were examined, the hydrophilic–hydrophobic behavior of ZnO films changed following Hu et al. and similar studies in the literature [61,62,63]. As the density of VO increases, the CA decreases, and the samples become hydrophilic. As the number of cycles increases, the density of VO decreases, and the CA increases. This is attributed to the water molecules coordinating with the surface’s oxygen vacancies, and dissociative adsorption occurs on the surface [64].
Photocatalytic activity tests of thin ZnO films were carried out at room temperature under a solar simulator using two different models of pollutants: methyl orange (MO) and methylene blue (MB). Changes of methyl orange and methylene blue concentrations as a function of time are given in the graphics in Fig. 8 in the presence of uncoated, 5, 10, 15, 25, 35, and 60 nm of ZnO-coated fabrics. No significant change was observed in the solution concentrations due to the test performed with uncoated fabrics for both dye solutions. With the deposition of 5 nm of ZnO coating of the glass fabric, 56 and 30% degradation were recorded in the MB and MO solutions, respectively. For the 10-nm ZnO coating, 68 and 46% degradation were recorded for MB and MO, respectively. Thus, the 10-nm ZnO sample showed the highest degradation rate for the MO dye in this work. The best result recorded in the MB solution and all photocatalytic activity measurements was for the 15-nm ZnO-coated sample that showed 78% degradation for MB and 40% degradation for MO. The 25-nm ZnO thin film-coated sample showed 68% degradation for MB and 36% for MO. As the thickness of the ZnO films increased to 35 nm, degradation rates decreased to 48% for MB and 26% for MO. Finally, 39% degradation was observed for MB and 12% for MO with the presence of a 60-nm ZnO-coated sample. Among the thin film-coated samples, the lowest degradation recorded in both model contaminants was the 60-nm ZnO-coated sample. Notably, the theoretical mass of the total deposited film on the fibers is approximately 80 µg for 10-nm films. Considering that our MB concentration is one of the highest ones studied in the literature, the photocatalyst:dye mass ratio is very low [38]. This shows that the photocatalytic activity of the ZnO-coated fabrics is very high. Furthermore increasing the photocatalyst: dye mass ratio with the increasing ALD film thickness reduces the photocatalytic activity of the samples. This indicates that rather than the mass increase of the photocatalytic material structure and surface properties play a crucial role. Photocatalytic degradation kinetics were evaluated by plotting an ln(C/C0) graph versus light exposure time. The rate constant (k) was calculated from the slope of the graphs in Fig. 8c, d [38]. The resulting graphs show that the photodegradation of both dyes follows first-order kinetics. Based on the other analysis results, the following comments can be made: For methylene blue and methyl orange solutions, 10 and 15-nm samples, which show much better results than others, also offer the lowest contact angle values measured above. Wettability/contact angle, like photocatalytic activity, is a property related to the film’s surface, so it can be said that they are directly related to each other. The results of this study show that photocatalytic efficiency increases with the increase in wettability. The 15-nm ZnO sample shows the best photocatalytic activity (i.e., 78%) value in the measurements, p-type character, maximum mobility, minimum carrier concentration values, and one of the lowest defect concentrations measured from PL results. In line with these results, various material aspects play an essential role in photocatalytic activity. Unlike the literature that reported that increasing the thickness of ALD ZnO films increases the photocatalytic activity [40, 41], the fabric samples showed significantly different behaviors. We think all the results indicate an optimum point for the photocatalytic activity of the fabric samples that can be attributed to their higher surface area and more complex surface morphology.
4 Conclusion
This study investigated the crystal structure and defects, electrical properties, and surface properties of the ALD ZnO films on fabric surfaces and the effects of all these parameters on photocatalytic activity. As a result of the investigation, it was observed that the crystal area and average grain size increased due to the increase in the film thickness with the increased number of cycles. Therefore, it was noted that there was a decrease in the linear defects in the structure. According to the results of the PL analysis, it was observed that the density of the oxygen vacancy defects decreased with the increase of the film thickness until a specific thickness. Still, an increase in the density of the defects was noted after the thickness of 15 nm. The changes in the electrical properties and wettability of the films reveal that the surface properties of the films have completely changed with the ZnO thin-film coating. Changes in these properties also affected the photocatalytic activity values of the films. With the increase in wettability, the photocatalytic efficiency also increases. At the same time, the 15-nm sample with the best photocatalytic efficiency value has the maximum mobility value. It is understood from other characterization analyses that these properties are related to the presence of oxygen vacancy defects in the structure and show a transition point at around 15 nm of thickness. Up until this thickness sample shows a p-type character, unlike bulk ZnO materials. This transition leads to an immediate change in the photocatalytic activity of the films as the thicknesses increase. Thus, a certain amount of oxygen vacancy defects in the structure improves photocatalytic efficiency, electrical properties, and wettability; increasing the density of these defects reverses the situation.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
M.M. Khan, D. Pradhan, Y. Sohn, Nanocomposites for visible light-ınduced photocatalysis (Springer, Cham, 2017)
J. Zeng, S. Liu, J. Cai, L. Zhang, J. Phys. Chem. C 114, 7806 (2010)
G. Ren, H. Han, Y. Wang, S. Liu, J. Zhao, X. Meng, Z. Li, Nanomaterials 11, 1804 (2021)
A. Joseph, A. Vijayanandan, Inorg. Chim Acta 545, 121284 (2023)
X. Xu, L. Hu, N. Gao, S. Liu, S. Wageh, A.A. Al-Ghamdi, A. Alshahrie, X. Fang, Adv. Funct. Mater. 25, 445 (2015)
L. Zheng, S. Han, H. Liu, P. Yu, X. Fang, Small 12, 1527 (2016)
Y. Chen, L. Su, M. Jiang, X. Fang, J. Mater. Sci. Technol. 105, 259 (2022)
S. Liu, L. Zheng, P. Yu, S. Han, X. Fang, Adv. Funct. Mater. 26, 3331 (2016)
J. Joe, H. Yang, C. Bae, H. Shin, Catalysts 2019(9), 149 (2019)
H.I. Akyildiz, E. Yigit, A.B. Arat, S. Islam, J. Photochem. Photobiol. A Chem. 448, 115308 (2024)
M. Ahmad, E. Ahmed, Y. Zhang, N.R. Khalid, J. Xu, M. Ullah, Z. Hong, Curr. Appl. Phys. 13, 697 (2013)
M. Fu, Y. Li, S. Wu, P. Lu, J. Liu, F. Dong, Appl. Surf. Sci. 258, 1587 (2011)
J. Gao, S. Jia, J. Liu, Z. Sun, X. Yang, D. Tang, J. Mater. Res. 36, 1538 (2021)
M.E. Borges, M. Sierra, E. Cuevas, R.D. García, P. Esparza, Sol. Energy 135, 527 (2016)
H. Chang, Water Res. 34, 407 (2000)
O. Sacco, V. Vaiano, L. Rizzo, D. Sannino, J. Clean. Prod. 175, 38 (2018)
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S. Sivaramakrishnan, J. Photochem. Photobiol. B. 190, 8 (2019)
N.M. Mahmoodi, M. Arami, J. Zhang, J. Alloys Compd. 509, 4754 (2011)
M. Zulfakar, N.A.H. Hairul, H.M.R. Akmal, M.A. Rahman, J. Appl. Sci. 11, 2320 (2011)
R. Oblak, M. Kete, U.L. Štangar, M. Tasbihi, J. Water Process. Eng. 23, 142 (2018)
K.L. Stano, S. Faraji, O. Yildiz, H. Akyildiz, P.D. Bradford, J.S. Jur, RSC Adv. 7, 27923 (2017)
S. Faraji, K. Stano, H. Akyildiz, O. Yildiz, J.S. Jur, P.D. Bradford, Nanotechnology 29, 295602 (2018)
K.L. Stano, S. Faraji, R. Hodges, O. Yildiz, B. Wells, H.I. Akyildiz, J. Zhao, J. Jur, P.D. Bradford, Small 12, 2432 (2016)
N. Miranda-García, S. Suárez, B. Sánchez, J.M. Coronado, S. Malato, M.I. Maldonado, Appl. Catal. B 103, 294 (2011)
H. Zhu, Z. Chen, Y. Hu, L. Gong, D. Li, Z. Li, J. Hazard. Mater. 389, 122119 (2020)
X. Kang, R. Floyd, S. Lowum, M. Cabral, E. Dickey, J. Maria, J. Am. Ceram. Soc. 102, 4459 (2019)
L. Rozenberga-Voska, J. Grabis, Solid State Phenom. 267, 3 (2017)
S. Pereira, A. Gonçalves, N. Correia, J. Pinto, L. Pereira, R. Martins, E. Fortunato, Sol. Energy Mater. Sol. Cells 120, 109 (2014)
D. Byun, Y. Jin, B. Kim, J. Kee Lee, D. Park, J. Hazard. Mater. 73, 199 (2000)
H.I. Akyildiz, E. Yigit, A.B. Arat, S. Islam, J. Photochem. Photobiol. A Chem. 448, 115308 (2024)
C. Huang, Y. Ding, Y. Chen, P. Li, S. Zhu, S. Shen, J. Environ. Sci. 60, 61 (2017)
P. Sun, R. Xue, W. Zhang, I. Zada, Q. Liu, J. Gu, H. Su, Z. Zhang, J. Zhang, D. Zhang, Catal. Today 274, 2 (2016)
S. Fukugaichi, ACS Omega 4, 15175 (2019)
M. Leskelä, M. Ritala, Thin Solid Films 409, 138 (2002)
R.L. Puurunen, Chem. Vap. Depos. 9, 249 (2003)
S.M. George, Chem. Rev. 110, 111 (2009)
H.I. Akyildiz, S. Diler, S. Islam, J. Vac. Sci. Technol. A 39, 022405 (2021)
S. Islam, H.I. Akyildiz, J. Mater. Sci. Mater. Electron. 32, 27027 (2021)
S. Islam, H.I. Akyildiz, J. Mater. Sci. Mater. Electron. 33, 18002 (2022)
A. Di Mauro, M.E. Fragalà, V. Privitera, G. Impellizzeri, Mater. Sci. Semicond. Process. 69, 44 (2017)
A. Di Mauro, M. Cantarella, G. Nicotra, V. Privitera, G. Impellizzeri, Appl. Catal. B 196, 68 (2016)
H.I. Akyildiz, B.A. Yilmaz, S. Diler, Fibers Polym 23, 2769 (2022)
H.K. Kaplan, A. Olkun, S.K. Akay, S. Pat, Opt. Quantum Electron. 53, 1 (2021)
M. Polat Gonullu, M. Soyleyici Cergel, H.I. Efkere, H. Ates, J. Mater. Sci. Mater. Electron. 32, 12059 (2021)
S.H.K. Park, Y.E. Lee, J. Mater. Sci. 39, 2195 (2004)
J. Iqbal, A. Jilani, P.M. Ziaul Hassan, S. Rafique, R. Jafer, A.A. Alghamdi, J. King Saud. Univ. Sci. 28, 347 (2016)
Y. Wang, K.-M. Kang, M. Kim, H.-H. Park, Appl. Surf. Sci. 529, 147095 (2020)
Y.-M. Chang, M.-L. Lin, T.-Y. Lai, H.-Y. Lee, C.-M. Lin, Y.-C.S. Wu, J.-Y. Juang, ACS Appl. Mater. Interfaces 4, 6676 (2012)
K. Vanheusden, W.L. Warren, C.H. Seager, D.R. Tallant, J.A. Voigt, B.E. Gnade, J. Appl. Phys. 79, 7983 (1996)
Y.-P. Wang, W.-I. Lee, T.-Y. Tseng, Appl. Phys. Lett. 69, 1807 (1996)
J.D. Ye, S.L. Gu, F. Qin, S.M. Zhu, S.M. Liu, X. Zhou, W. Liu, L.Q. Hu, R. Zhang, Y. Shi, Y.D. Zheng, Appl. Phys. A 81, 759 (2005)
J.L. Tian, H.Y. Zhang, G.G. Wang, X.Z. Wang, R. Sun, L. Jin, J.C. Han, Superlattices Microstruct. 83, 719 (2015)
M. Bouderbala, S. Hamzaoui, B. Amrani, A.H. Reshak, M. Adnane, T. Sahraoui, M. Zerdali, Phys. B Condens. Matter. 403, 3326 (2008)
J. Yang, A. Bahrami, X. Ding, S. Lehmann, N. Kruse, S. He, B. Wang, M. Hantusch, K. Nielsch, Adv. Mater. Interfaces 9, 2101953 (2022)
C. Hong, K.-M. Kang, M. Kim, Y. Wang, T. Kim, C. Lee, H.-H. Park, Mater. Sci. Eng. B 273, 115401 (2021)
A. Janotti, C.G. Van de Walle, Phys. Rev. B 76, 165202 (2007)
Ü. Özgür, Y.A.I. Alivov, C. Liu, A. Teke, M.A. Reshchikov, S. Doğan, V. Avrutin, S.-J. Cho, H. Morkoç, J. Appl. Phys. 98, 041301 (2005)
Y. Wang, K.-M. Kang, M. Kim, H.-H. Park, Thin Solid Films 660, 852 (2018)
J. Li, Q. Sun, S. Han, J. Wang, Z. Wang, C. Jin, Prog. Org. Coat. 87, 155 (2015)
K. Yadav, B.R. Mehta, S. Bhattacharya, J.P. Singh, Sci. Rep. 6, 35073 (2016)
H. Hu, H.-F. Ji, Y. Sun, Phys. Chem. Chem. Phys. 15, 16557 (2013)
J. Wu, J. Chen, J. Xia, W. Lei, B. Wang, Adv. Mater. Sci. Eng. 2013, 1 (2013)
L. Huang, S.P. Lau, H.Y. Yang, E.S.P. Leong, S.F. Yu, S. Prawer, J. Phys. Chem. B 109, 7746 (2005)
R.-D. Sun, A. Nakajima, A. Fujishima, T. Watanabe, K. Hashimoto, J. Phys. Chem. B 105, 1984 (2001)
Acknowledgements
The authors express their gratitude for the partial financial support received from the Scientific and Technological Research Council of Turkey (TUBITAK) through Grants # 118M617 and 218M275, as well as from Bursa Uludag University through Grant # FGA-2023-1309. The authors sincerely thank Prof. Omer Y. Gumus from Bursa Technical University for his invaluable assistance with the UV–Vis analysis of the films. Furthermore, the authors wish to acknowledge the contributions of Prof. Sertan Kemal Akay and his research group for their valuable assistance with the Hall Effect measurements.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The Scientific and Technological Research Council of Turkey (TUBITAK), [Grant Numbers 118M617 and 218M275] and Bursa Uludag University, [Grant # FGA-2023-1309].
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arat, A.B., Akyildiz, H.I. A mechanistic approach to determine the relationship between film structure, electronic properties, and photocatalytic activity of ALD ZnO thin films on glass gibers. J Mater Sci: Mater Electron 35, 1066 (2024). https://doi.org/10.1007/s10854-024-12843-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12843-7