Abstract
Organic molecule stabilized face-centered cubic structured bismuth fluoride (BiF3) nanoparticles (NPs), space group: Fm-3 m, were prepared using a wet chemical synthesis route for symmetric supercapacitor application. For freestanding three electrode system, at current density (Cd) 3.3 A.g−1, the material displayed a maximum specific capacitance (SCp) value 717.3 F.g−1. The symmetric device based on BiF3 attained a maximum specific capacity (SC) value of 228.4 mAh.g−1 at 0.06 A.g−1. At the current density of 0.30 A.g−1, the device displayed the energy density (Ed) and power density (Pd) values of 0.17 Wh.kg−1 and 343.7 W.kg−1, respectively. Furthermore, the fabricated symmetric supercapacitor displayed 90% of capacity retention and 95% of coulombic efficiency at 0.12 A.g−1 for 1000 galvanostatic charge–discharge (GCD) cycles. The symmetric supercapacitor based on BiF3 NPs exhibited excellent electrochemical performance, which could be an appropriate choice for application in different integrated energy storage systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is a multidisciplinary area of research in modern science and technology that includes a novel synthesis of nanostructured materials with diverse applications. Nanostructured materials hold a great deal of possibilities for several applications in photonics, nonlinear optics, biological sensors, optoelectronics, electronics, and energy storage [1,2,3,4,5,6,7]. Functional nanomaterials exhibit important role in energy production and energy storage applications due to their excellent electron transport ability and chemical stability.
Transition metal oxides are known to be an excellent electrode materials due to their different valence states and thermal stability. Materials like Fe3O4, MnO2, NiO, and RuO2 possess good conductivity and good capacitance value [8,9,10,11]. Nanostructured transition metal sulfides have shown efficiency as electrode material for high-performance supercapacitor application. They offer numerous advantages, including higher electrical conductivity and storage capacity, good redox characteristics, high specific capacitance, quicker ion diffusion and long cycle life [12,13,14].
Beside transition materials, compounds based on bismuth have shown excellent rate performance and high capacitance [15, 16]. Thin film of Bi2O3, synthesized via electrodeposition technique, was reported for supercapacitor application with the SCp value of 98 F.g−1 in aqueous NaOH solution [17]. In the presence of KOH electrolyte (1.0 mol dm−3), nanosized Bi2O3, supported by organic molecules, has been utilized as an electrode material for supercapacitor application. It showed a SCp value of 407 F.g−1 with an Ed value 113 Wh.kg−1 at 1.0 A.g−1 [18]. Bi2S3 NPs synthesized using NH2CSNH2 and Na2S, as sulphur sources, exhibited maximum SCp values of 250 F.g−1 and 491 F.g−1, respectively, at 0.5 A.g−1 [19]. Bi3Mn2O7 NPs exhibited supercapacitive performance with SCp value of 612 F.g−1 at 1 A.g−1, under alkaline condition [20]. Nanosized Bi2WO6, synthesized by sonochemical method, exhibited a SCp of 608 F.g−1 in KOH (1.0 mol dm−3) at 0.5 mA.cm−2 (Cd) [21]. Perovskites of bismuth ferrite also displayed supercapacitive property with high cycling stability and rate capability [22, 23]. Bismutite (Bi2O2CO3) is a multifunctional material and have the potential to perform as an electrode in supercapacitor applications [24]. Copper bismuth oxide based NPs displayed a maximum SC value of 144 mAh.g−1 at 1.0 A.g−1 with long-term cycling stability [25]. An electrodeposition process was reported to fabricate bismuth molybdate nanowires that displayed a high value of SCp (1075 F.g−1) at 0.1 A.g−1 [26]. Supercapacitor electrode based on nanostructured bismuth oxyiodide (BiOI/Bi9I2) displayed SCp of 515.5 F.g−1. A symmetric device based on the above material exhibited Ed and Pd values of 38.2 Wh.kg−1 and 2280.4 W.kg−1, respectively, at 2 A.g−1 [27]. Metal-halide perovskite, methyl ammonium bismuth triiodide, have been reported for photo-supercapacitor application with the maximum areal capacitance value of 5.5 mF.Cm−2 [28].
Nanostructured metal fluorides have gained enormous attention owing to their interesting application in energy storage. It is reported that the capacitive behavior of MnF2 and CoF2 NPs enables them to be used as electrodes in supercapacitor application [29]. CoF2 NPs loaded carbon cloth, a flexible composite, was reported as a cathode material for Li-ion battery that exhibited a high SC of 330 mAh.g−1 at 100 mA.g−1 [30].
Limited number of reports are found in the literature based on bismuth fluoride for electrochemical energy storage applications [31, 32]. This is a first kind of report and marks as a significant milestone for the utilization of bismuth fluoride within the domain of symmetric supercapacitor applications. In this study, bismuth carbonate was applied as a precursor of bismuth to synthesis BiF3 NPs. The product was characterized by microscopic, structural and optical techniques and further applied as an electrode material for symmetric supercapacitor application.
2 Experimental section
2.1 Materials
All the reagents, bismuth carbonate, aniline, ammonium fluoride (NH4F), poly-vinylidene fluoride (PVDF), carbon black (CB) and N-methyl pyrrolidine (NMP), were of analytical grade and applied without any further purification.
2.2 Synthesis of bismuth fluoride nanoparticles
For a synthesis process, methanolic solution of aniline (5:1 ratio) was drop wise added to bismuth carbonate (0.06 g) and subsequently Bi(III)-aniline complex was formed. The complex material was mixed with an aqueous solution of NH4F (5.0 mL, 0.3 mol.dm−3) and left for six hours at room temperature (27°C) under stirring condition. The synthesized solid product was recovered by filtration, dried at 60°C under vacuum and characterized using various analytical techniques. The final product (bismuth fluoride, BiF3) was further applied as the electrode material for three-electrode (freestanding) and two electrode (coin cell) systems for supercapacitor application.
2.3 Fabrication of three-electrode system
For the preparation of freestanding electrode, a slurry was made using BiF3 (active material), PVDF and CB with the ratio of 8:1:1 (by weight), respectively, in presence of NMP, as a solvent. The prepared slurry (0.6 mg) was coated on a Ni-foam, acted as a working electrode. Aqueous KOH solution (1.0 mol.dm−3) was used as an electrolyte. A saturated calomel electrode and Pt wire were acted as the reference and counter electrodes, respectively [33].
2.4 Fabrication of symmetric device
BiF3 based slurry (3.2 mg), prepared as above, was spread over two identical piece of Ni-foam, separately, and treated at 60°C for 12 h. A piece of filter paper, soaked with KOH solution (1.0 mol.dm−3), was placed between two Ni-foams and inserted into a coin cell. The fabrication of the symmetric coin cell device is illustrated in the supporting information, figure S1. The electrochemical properties of both freestanding electrode and symmetric device were evaluated using a potentiostat, Biologic SP300. All the calculations required to determine the electrochemical parameters are mentioned in the supporting information.
3 Result and discussion
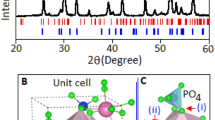
The transmission electron microscope, JEOL (JEM-2100) analytical TEM, image of BiF3 NPs is shown in Fig. 1A. The dark spots are the BiF3 particles within the size range of 5–15 nm. Furthermore, the image revealed that the majority of the particles are spherical in shape.
A Transmission electron microscopy image of organic molecule stabilized bismuth fluoride nanoparticles. B X-ray diffraction pattern of bismuth fluoride nanoparticles; indexed according face centered cubic structure (JCPDS: 731988, blue colour bar). The unit cell representation of bismuth fluoride projected along c-axis, inset figure. C Raman spectra of bismuth fluoride measured within the range from 50 to 550 cm−1
The X-ray diffraction pattern of BiF3 particles, Fig. 1B, was collected using Philips PANalytical X’pert diffractometer with Cu-Kα radiation. The observed pattern (red line) was indexed according to the face centered cubic structure (JCPDS: 731988) and belongs to the space group of Fm-3 m (225). Previously reported Rietveld refinement analysis of BiF3 system confirmed the formation of cubic lattice symmetry with the value of a = b = c = 5.820 Å and bond length (Bi-F) between ~ 2.52–2.92 Å [34]. The diffraction peaks revealed the crystallinity of the synthesized material. The unit cell structure of BiF3 is represented in Fig. 2A, projected along c-axis. The structure is made up of a symmetric Bi-F polyhedra network with the Bi ion the centre [35].
A Cyclic voltammogram of bismuth fluoride under different scan rates for the three electrode system. B Galvanostatic charge–discharge profile of bismuth fluoride under different current densities. Specific capacitance as a function of C scan rate (mV.s−1) and D current density (A.g−1). E Capacitance retention and coulombic efficiency for 1000 cycles, at a current density of 18.3 A.g1. Inset figure shows the initial and final charge–discharge cycles
Raman spectrum of BiF3 NPs was analyzed using Jobin–Yvon T64000 spectrometer with an argon ion laser source of 514.5 nm within the frequency range from 30 to 550 cm−1, Fig. 1C.
The spectrum was deconvoluted (red line) into six active modes (ν1 – ν6) positioned at ν1 ~ 71 cm−1, ν2 ~ 90.1 cm−1, ν3 ~ 109.2 cm−1, ν4 ~ 157.0 cm−1, ν5 ~ 219 cm−1 and ν6 ~ 435 cm−1. The low frequency Raman active modes (ν1, ν2 and ν3) are originated due to the Bi-F bond vibration, which is similar to the other reported metal halide based systems [36]. The intense Raman active mode positioned ~ 157.0 cm−1 (ν4) is related to vibration of poly-fluoride and F-F bond interaction. Towards higher frequency side, symmetrical stretching mode of Bi-F and Bi-F-Bi networks are responsible for low intensity diffuse modes positioned at ν5 and ν6 in bismuth fluoride system [36, 37].
3.1 Electrochemical analysis for three electrode system
Cyclic voltammetry (CV) and GCD techniques were applied to evaluate the electrochemical properties of the freestanding electrode. Figure 2A, displays the voltammograms within the scan rates from 15 to 50 mV.s−1 (main panel) and from 4 to 10 mV.s−1 (inset). The presence of different redox peaks in all the voltammograms indicate the pseudocapacitive behaviour of the electrode [32]. With increasing scan rate, a gradual increase of Cd was noticed, Fig. 2A. At the scan rate of 4.0 and 50 mV.s−1, the values of Cd were achieved 12.3 and 56.08 A.g−1, respectively, for the anodic peak current. Figure 2B demonstrates the GCD profile within the voltage range from −0.9 V to −0.1 V and the current density ranging from 3.3 to 25 A.g−1. At 3.3 A.g−1, the maximum SCp for the BiF3 modified electrode was 717.3 F.g−1. In the GCD study, the charge curves are not symmetric as compared to the corresponding discharge curves, implies pseudocapacitive behaviour. The values of SCp obtained from the CV and GCD curves are mentioned in Table 1, calculated from the Eq. 1 & 2, supporting information. The graphical representation, Fig. 2 (C and D), display the SCp as a function of scan rate and Cd, respectively. The maximum SCp values 745.3 F.g−1 and 717.3 F.g−1 were obtained at the scan rate of 4 mV.s−1 and at the current density of 3.3 A.g−1, respectively. Low current density and low scan rate resulted a high value of capacitance, which may be due to longer exposure time of the electrolyte ions with the active electrode material. Figure 2E shows the capacitance retention and coulombic efficiency for 1000 cycles, at 18.3 A.g−1. The capacitance retention maintained a steady trend for the first few hundred cycles and then decreased, possibly due to the mechanical fatigue of the material. Retention of capacitance value of 97% was achieved at the end of the 1000 cycles, with a coulombic efficiency of 98%. It is worth to note that the shape of the charge–discharge curves remained almost similar for the first and 1000th cycle, confirming high stability of the electrode, Fig. 2E, inset.
3.2 Electrochemical analysis of symmetric device
Figure 3A shows the voltammograms of the symmetric device within the voltage window from 0.0 to 1.1 V under the scan rate from 20 to 160 mV.s−1 in presence of KOH (1 mol.dm−3). The CV profile shows quasi-rectangular pattern, indicating the pseudocapacitive behaviour of the device. The GCD study of the device was carried out under different current density (Cd), ranging from 0.06 to 0.30 A.g−1, Fig. 3B. The device exhibited non-triangular pattern of charge–discharge (CD) curves, indicating reversible a redox process for charge storage mechanism. The maximum SC obtained for the device was 228.4 mAh.g−1, at 0.06 A.g−1.
A Cyclic voltammograms of the symmetric device within the voltage range from 0.0 to 1.1 V under different scan rates. B Galvanostatic charge–discharge profile of the device under different current densities. Specific capacity as a function of C scan rate (mV.s−1) and D current density (A.g−1). E Capacity retention and coulombic efficiency for 1000 cycles, at a current density of 0.12 A.g−1. Inset figure shows the charge–discharge profile of the device, for the first and last five cycles
Figure 3, C & D, illustrate the SC as a function of scan rate and Cd, respectively. The maximum SC values 228.4 and 290.7 mAh.g−1 were achieved at 0.06 A.g−1 (current density) and 4 mV.s−1 (scan rate), respectively. The CD performance of the device was tested for 1000 cycles at 0.12 A.g−1. After 1000 CD cycles, the capacity retention and coulombic efficiency were obtained 90% and 95%, respectively, indicating high stability of the device, Fig. 3E. The stable CD cycles, for the first and last five cycles, indicates the high durability of the device, Fig. 3E, inset. The graphical representation displays the Ed and Pd with respect to Cd of the device, Fig. 4A. At 0.30 A.g−1, the device exhibited Ed and Pd values of 0.17 Wh.kg−1 and 343.7 W.kg−1, respectively, whereas at 0.06 A.g−1 (low Cd) the device displayed Ed and Pd of 1.25 Wh.kg−1 and 68.7 W.kg−1, respectively. The bismuth fluoride based device was charged for 25 s under the current density value of 0.31 A.g−1 to light up a green LED (light-emitting diode). The intensity of the light is displayed in figure S2, supporting information, (A) at the beginning and (B) at the end of 60 s (discharging period).
A The bar graph represents the energy and power density as a function of current density of the bismuth fluoride based symmetric device. Graphical representation of B inverse of specific capacity as a function of scan rate (v1/2) and C specific capacity as a function of inverse of scan rate (v−1/2). D Contribution of electrical double layer capacitance (capacitive) and pseudocapacitance (diffusive) towards total specific capacity of the device
The capacitive and diffusive contributions of the device were used to evaluate the charge storage mechanism. We have performed the CV measurement under various scan rates from 20 to 160 mV.s−1, Fig. 3A, to differentiate the contributions of different reaction mechanisms. Both electric double-layer capacitance (capacitive) and pseudocapacitance (diffusive) contribute to the specific capacity of device. The Trassati method was applied to evaluate the capacitive contribution and diffusion-controlled capacity from the experimental CV data [38]. The total charge stored within the material (inner and outer surface) is calculated from the Trassati plot. The equation, \({C}_{T}= {C}_{dl}+ {C}_{pc}\), determines the total charge stored (\({C}_{T}\)) via the surface redox process and charge accumulation between active material and electrolyte, which consists of double layer capacitance (\({C}_{dl}\)) and pseudocapacitance (\({C}_{pc}\)). The total capacity contribution was estimated by the equation, \({C}^{-1}={k}_{1}{(v}^{0.5})+ {C}_{T}\), where \(v\) is the scan rate and \({C}^{-1}\) is the inverse of specific capacity obtained from the voltammograms. From the linear fitting of \({C}^{-1}\) versus \({v}^{0.5}\), Fig. 4B, the total capacity (\({C}_{T}\)) can be evaluated. The amount of charge stored at the outer surface was calculated by extrapolating the plot \(C\) versus \({v}^{-0.5}\), Fig. 4C, equation: \(C={k}_{2} {v}^{-0.5}+ {C}_{dl}\). Figure 4D exhibits the capacitive (7.7%) and diffusive (92.2%) contribution of the device, calculated from the Eq. (6 and 7), supporting information.
The electrical property of the symmetric device was studied using the electrochemical impedance spectroscopy technique to determine the interaction between electrode–electrolyte interface within the frequency range from 100 mHz to 200 kHz. Figure 5A displays the Nyquist plot of the device with the absence of a high frequency semi-circle, indicating a low charge transfer resistance, denotes the capacitive behavior [39]. Additionally, in the low frequency range, the Nyquist plot exhibits a quasi-vertical line parallel to the imaginary axis (Z″), indicating a capacitive method of energy storage [39]. Figure 5A, inset (I), displays a magnified image of the Nyquist plot for the high frequency region. In high-frequency region, the intercept of the Z’-axis denotes equivalent series resistance of the device. This resistance is the combination of interfacial contact resistance between the active material and current collector, internal resistance of the active material, and electrolyte ion resistance [39]. The equivalent circuit model, extracted from the Nyquist plot, is presented in Fig. 5A, inset (II). Within the circuit, the equivalent series resistance (R1) is coupled in parallel with the constant phase element (Q2) and in series with the charge transfer resistance (R2). The leakage current resistance (R3) is coupled in series with the Warburg-frequency (W) and in parallel with the leakage capacitance (Q3). All the fitting parameters extracted from the equivalent circuit models are included in table S1, supporting information. The capacitive behaviour of the device was further confirmed by the bode plot with the phase angle of −83.1°, Fig. 5B, which is close to the ideal capacitor [40]. The real capacitance (C′) value was achieved 1.1 mF, Fig. 5C, which is the deliverable capacitance of the device. The imaginary capacitance (C′′) reveals the energy loss through an irreversible mechanism [41, 42]. Figure 5C shows the graphical representation of imaginary capacitance with respect to frequency. A peak was appeared at 7.2 Hz, which correspond to the relaxation time of 24 ms, indicate the time required to charge the device [43].
A comparison databased on supercapacitive performance of some previously reported bismuth based materials is included in Table 2 [44,45,46,47,48,49,50,51,52].
4 Conclusion
Bismuth fluoride nanoparticles within the size range of 5–15 nm were synthesized using a one-pot complexation-mediated method. The X-ray diffraction pattern of bismuth fluoride confirmed the formation face centered cubic structure with the space group of Fm-3 m (225). The Raman spectrum revealed the various Bi-F bond vibrations originated from the low frequency Raman active modes. The synthesized bismuth fluoride was applied as electrode material for supercapacitor application. For three electrode system, the maximum specific capacitance values 745.3 F.g−1 and 717.3 F.g−1 were obtained at the scan rate of 4 mV.s−1 and at the current density of 3.3 A.g−1, respectively. A symmetric device was fabricated based on bismuth fluoride that exhibited maximum specific capacity value of 228.4 mAh.g−1 at the current density of 0.06 A.g−1. The device exhibited the energy and power density values of 0.17 Wh.kg−1 and 343.7 W.kg−1, respectively, at the current density of 0.30 A.g−1. The device stability test, performed for 1000 charge–discharge cycles under the current density of 0.12 A.g−1, resulted in 90% capacity retention and 95% coulombic efficiency. Due to the above mentioned performances, the device could be a potential candidate for real-life applications.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
V.K. Perla, S.K. Ghosh, K. Mallick, Ultrafine nickel sulfide-based bipolar resistive switching device as artificial synapses for neuromorphic application. ACS Appl. Electron. Mater. 4, 6117–6124 (2022)
H. Kim, S. Beack, S. Han, M. Shin, T. Lee, Y. Park, K.S. Kim, A.K. Yetisen, S.H. Yun, W. Kwon, S.K. Hahn, Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv. Mater. 30, 1701460 (2018)
Y. Zhanga, Y. Wang, Nonlinear optical properties of metal nanoparticles: a review. RSC Adv. 7, 45129–45144 (2017)
M. Lee, H. Seung, J.I. Kwon, M.K. Choi, D.H. Kim, C. Choi, Nanomaterial-based synaptic optoelectronic devices for sensor preprocessing of image data. ACS Omega 8, 5209–5224 (2023)
N.P. Shetti, S.D. Bukkitgar, K.R. Reddy, C.V. Reddy, T.M. Aminabhavi, ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 141, 111417 (2019)
V.K. Perla, S.K. Ghosh, K. Mallick, Oxygen vacancy-controlled dielectric relaxation and field-driven polarization of bismuth-manganese bimetallic oxide nanoparticles: implications for capacitors. ACS Appl. Nano Mater. 4, 7116–7124 (2021)
K. Das, D. Majumdar, Prospects of MXenes-graphene nanocomposites for advanced supercapacitor applications. J. Electroanal. Chem. 905, 115973 (2021)
V.D. Nithya, N.S. Arul, Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A. 4, 10767–10778 (2016)
I.I. Misnon, R.A. Aziz, N.K.M. Zain, B. Vidhyadharan, S.G. Krishnan, R. Jose, High performance MnO2 nanoflower electrode and the relationship between solvated ion size and specific capacitance in highly conductive electrolytes. Mater. Res. Bull. 57, 221–230 (2014)
F. Liu, X. Wang, J. Hao, S. Han, J. Lian, Q. Jiang, High density arrayed Ni/NiO core-shell nanospheres evenly distributed on graphene for ultrahigh performance supercapacitor. Sci. Rep. 7, 17709 (2017)
D. Susanti, D. Tsai, Y. Huang, A. Korotcov, W. Chung, Structures and electrochemical capacitive properties of RuO2 vertical nanorods encased in hydrous RuO2. J. Phys. Chem. C 111, 9530–9537 (2007)
J. Rehman, K. Eid, R. Ali, X. Fan, G. Murtaza, M. Faizan, A. Laref, W. Zheng, R. Varma, Engineering of transition metal sulfide nanostructures as efficient electrodes for high-performance supercapacitors. ACS Appl. Energy Mater. 5, 6481–6498 (2022)
W. Luo, G. Zhang, Y. Cui, Y. Liu, C. Jin, J. Hao, J. Zhanga, W. Zheng, One-pot synthesis of highly stable carbon–MoS2 nanosphere electrodes using a co-growth mechanism for supercapacitors. New J. Chem. 42, 10111–10117 (2018)
Y. Hanifehpour, M. Abdolmaleki, N. Moradi, A.H.S. Farhood, S. Ahmadiyeh, G.R. Allahgholipoura, J. Hosseinid, Investigation of the supercapacitor behavior of MoS2 and Fe-doped MoS2 nano-flowers synthesized using the hydrothermal method. New J. Chem. 46, 20932–20939 (2022)
B. Sarma, A.L. Jurovitzki, Y.R. Smith, S.K. Mohanty, M. Misra, Redox-induced enhancement in interfacial capacitance of the titania nanotube/bismuth oxide composite electrode. ACS Appl. Mater. Interfaces 5, 1688–1697 (2013)
H.W. Wang, Z.A. Hu, Y.Q. Chang, Y.L. Chen, Z.Q. Lei, Z.Y. Zhang, Y.Y. Yang, Facile solvothermal synthesis of a graphene nanosheet–bismuth oxide composite and its electrochemical characteristics. Electrochim. Acta 55, 8974–8980 (2010)
T.P. Gujar, V.R. Shinde, C.D. Lokhande, S.H. Han, Electrosynthesis of Bi2O3 thin films and their use in electrochemical supercapacitors. J. Power. Sources 161, 1479–1485 (2006)
N. Devi, S. Ghosh, S.C. Ray, K. Mallick, Organic matrix stabilized ultra-fine bismuth oxide particles for electrochemical energy storage application. ChemistrySelect 3, 12057–12064 (2018)
N. Devi, S. Ghosh, K. Mallick, Supercapacitive performance of highly dispersed bismuth sulfide nanoparticles in organic matrix: the role of sulphur source. Inorg. Chem. Commun. 103, 93–99 (2019)
N. Devi, S.K. Ghosh, V.K. Perla, K. Mallick, Organic-inorganic complexation chemistry mediated synthesis of bismuth-manganese bimetallic oxide for energy storage application. ACS Omega 5, 18693–18699 (2020)
V.D. Nithya, R.K. Selvan, D. Kalpana, L. Vasylechko, C. Sanjeeviraja, Synthesis of Bi2WO6 nanoparticles and its electrochemical properties in different electrolytes for pseudocapacitor electrodes. Electrochim. Acta 109, 720–731 (2013)
C.D. Lokhande, T.P. Gujar, V.R. Shinde, R.S. Mane, S.H. Han, Electrochemical supercapacitor application of perovskite thin films. Electrochem. commun. 9, 1805–1809 (2007)
V.V. Jadhav, M.K. Zate, S. Liu, M. Naushad, R.S. Mane, K.N. Hui, S.H. Han, Mixed-phase bismuth ferrite nanoflake electrodes for supercapacitor application. Appl. Nanosci. 6, 511–519 (2016)
T. Selvamani, B.G.S. Raj, S. Anandan, J.J. Wu, M.A. Kumar, Synthesis of morphology-controlled bismutite for selective applications. Phys. Chem. Chem. Phys. 18, 7768–7779 (2016)
A.A. Ensafi, N. Ahmadi, B. Rezaei, Electrochemical preparation of CuBi2O4 nanoparticles on nanoporous stainless steel as a binder-free supercapacitor electrode. J. Alloys Compd. 652, 39–47 (2015)
Z.-Q. Liu, L.-Y. Tang, N. Li, K. Xiao, J. Wang, J.-H. Zhang, Y.-Z. Su, Y.-X. Tong, Facile synthesis of large-area hierarchical bismuth molybdate nanowires for supercapacitor applications. J. Electrochem. Soc. 159, D582 (2012)
S. Park, N.M. Shinde, P.V. Shinde, D. Lee, J.M. Yun, K.H. Kim, Chemically grown bismuth-oxy-iodide (BiOI/Bi9I2) nanostructure for high performance battery-type supercapacitor electrodes. Dalton Trans. 49, 774–780 (2020)
J.K. Pious, M.L. Lekshmi, C. Muthu, R.B. Rakhi, C. Vijayakumar, Zero-dimensional methyl ammonium bismuth iodide-based lead free perovskite capacitor. ACS Omega 2, 5798–5802 (2017)
R. Ma, Y. Zhou, L. Yao, G. Liu, Z. Zhou, J.-M. Lee, J. Wang, Q. Liu, Capacitive behaviour of MnF2 and CoF2 sub-micron / nanoparticles synthesized via a mild ionic liquid-assisted route. J. Power. Sources 303, 49–56 (2016)
Q. Zhang, Y.T. Huang, X. Chen, A. Pan, Z. Cai, S. Liu, Y. Zhang, CoF2 nanoparticles grown on carbon fiber cloth as conversion reaction cathode for lithium-ion batteries. J. Alloys Compd. 805, 539–544 (2019)
J.F. Baumgärtner, F. Krumeich, M. Wörle, K.V. Kravchyk, M.V. Kovalenko, Thermal synthesis of conversion-type bismuth fluoride cathodes for high-energy-density Li-ion batteries. Commun. Chem. 5, 6 (2022)
N. Devi, S. Ghosh, V.K. Perla, T. Pal, K. Mallick, Laboratory based synthesis of the pure form of gananite (BiF3) nanoparticles: a potential material for electrochemical supercapacitor application. New J. Chem. 43, 18369–18376 (2019)
S. Sahoo, J.-J. Shim, Facile synthesis of three-dimensional ternary ZnCo2O4/ reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 5, 241–251 (2017)
S.K. Ghosh, V.K. Perla, K. Mallick, Enhancdement of dielectric and electric field induced polarization of bismuth fluoride nanoparticles within the layered strucutre of carbon nitride. Sci. Rep. 10, 14835 (2020)
O. Bodak, P. Demchenko, Y. Seropegin, A.O. Fedorchuk, Cubic structure types of rare earth intermetallics and related compounds. Z. Kristallogr. Cryst. Mater. 221, 482–492 (2006)
L.N. Ignatieva, N.V. Surovtsev, N.N. Savchenko, S.V. Adichtchev, E.B. Merkulov, Y.V. Marchenko, G.A. Zverev, V.M. Bouznik, Glasses in the system ZrF4-BaF2-BiF3: thermal and spectroscopic properties. J. Non Cryst. Solids 426, 7–12 (2015)
V.Y. Kavun, E.I. Voit, R.M. Yaroshenko, V.K. Goncharuk, Structure and ion mobility in glasses in the BiF3-PbF2-ZrF4 systems studied by raman and nmr spectroscopy. J. Non Cryst. Solids 401, 224–231 (2014)
S. Ardizzone, G. Fregonara, S. Trasatti, “Inner” and “Outer” active surface of RuO. Electrochim. Acta 35, 263–267 (1990)
T.S. Mathis, N. Kurra, X. Wang, D. Pinto, P. Simon, Y. Gogotsi, Energy storage data reporting in perspective-guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 9, 1902007 (2019)
M. Mahdavian, M.M. Attar, Another approach in analysis of paint coatings with EIS measurement: phase angle at high frequencies. Corros. Sci. 48, 4152–4157 (2006)
M.N. Rantho, M.J. Madito, F.O. Ochai-Ejeh, N. Manyala, Asymmetric supercapacitor based on vanadium disulfide nanosheets as a cathode and carbonized iron cations adsorbed onto polyaniline as an anode. Electrochim. Acta 260, 11–23 (2018)
P.L. Taberna, P. Simon, J.F. Fauvarque, Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 150, A292 (2003)
N.F. Sylla, N.M. Ndiaye, B.D. Ngom, B.K. Mutuma, D. Momodu, M. Chaker, N. Manyala, Ex-situ nitrogen-doped porous carbons as electrode materials for high performance supercapacitor. J. Colloid Interface Sci. 569, 332–345 (2020)
A.M. Teli, T.S. Bhat, S.A. Beknalkar, S.M. Mane, L.S. Chaudhary, D.S. Patil, S.A. Pawar, H. Efstathiadis, J.C. Shin, Bismuth manganese oxide based electrodes for asymmetric coin cell supercapacitor. Chem. Eng. J. 430, 133138 (2022)
A. Al-Osta, B.S. Samer, U.T. Nakate, V.V. Jadhav, R.S. Mane, Electrodeposited spruce leaf-like structured copper bismuth oxide electrode for supercapacitor application. Microelectron. Eng. 229, 111359 (2020)
M. Karnan, O.P. Nanda, L. Durai, S. Badhulika, One-step hydrothermal synthesis of Bi2CuO4 nanoflakes: an excellent electrode material for symmetric supercapacitors. J. Energy Storage 63, 106993 (2023)
N.M. Shinde, Q.X. Xia, J.M. Yun, S. Singh, R.S. Mane, K.-H. Kim, A binder-free wet chemical synthesis approach to decorate nanoflowers of bismuth oxide on Ni-foam for fabricating laboratory scale potential pencil-type asymmetric supercapacitor device. Dalton Trans. 46, 6601–6611 (2017)
R. Liu, L. Ma, G. Niu, X. Li, E. Li, Y. Bai, G. Yuan, Oxygen-deficient bismuth oxide/graphene of ultrahigh capacitance as advanced flexible anode for asymmetric supercapacitors. Adv. Funct. Mater. 27, 1701635 (2017)
N.M. Shinde, Q.X. Xia, J.M. Yun, R.S. Mane, K.H. Kim, Polycrystalline and mesoporous 3-d bi2o3 nanostructured negatrodes for high-energy and power-asymmetric supercapacitors: superfast room-temperature direct wet chemical growth. Appl. Mater. Interfaces 10, 11037–11047 (2018)
P.V. Shinde, N.M. Shinde, J.M. Yun, R.S. Mane, K.H. Kim, Facile chemical synthesis and potential supercapattery energy storage application of hydrangea-type Bi2MoO6. ACS Omega 4, 11093–11102 (2019)
Y. Ma, Y. Jia, L. Wang, M. Yang, Y. Bi, Y. Qi, Facile growth of Bi2MoO6 nanosheet arrays on Ni foam as an electrode for electrochemical applications. RSC Adv. 6, 12093–12099 (2016)
J. Xu, Z. Meng, Z. Hao, X. Sun, H. Nan, H. Liu, Y. Wang, W. Shi, H. Tian, X. Hu, Oxygen-vacancy abundant alpha bismuth oxide with enhanced cycle stability for high-energy hybrid supercapacitor electrodes. J. Colloid Interface Sci. 609, 878–889 (2022)
Funding
Open access funding provided by University of Johannesburg. This study was financially supported by the Faculty of Science, University Research Council and the Global Excellence and Stature program 4.0, University of Johannesburg.
Author information
Authors and Affiliations
Contributions
PK: Methodology, investigation, supercapacitor studies, draft writing, SKG: Material characterization, supercapacitor studies, CS: Material characterization, KM: Supervision, funding acquisition, conceptualization, writing.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumari, P., Ghosh, S.K., Saha, C. et al. One pot synthesis of nanostructured bismuth fluoride tailored for symmetric supercapacitor performance. J Mater Sci: Mater Electron 35, 1034 (2024). https://doi.org/10.1007/s10854-024-12820-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12820-0